SML0038
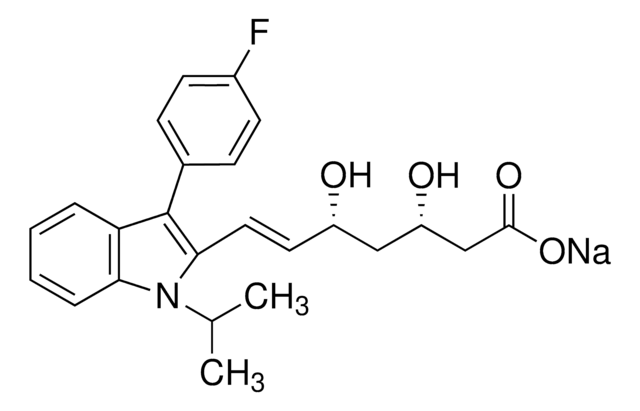
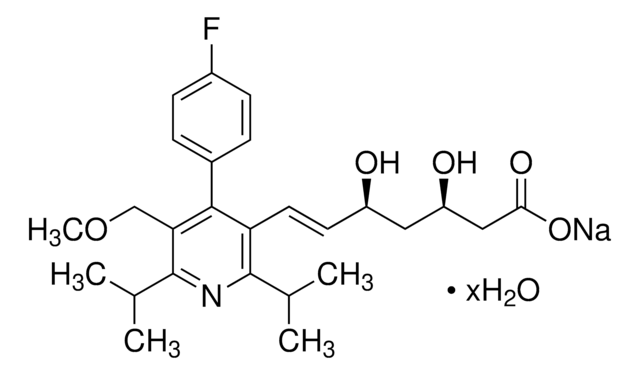
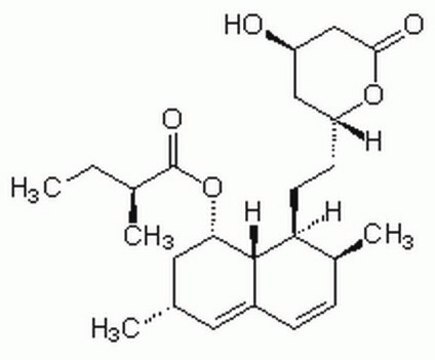
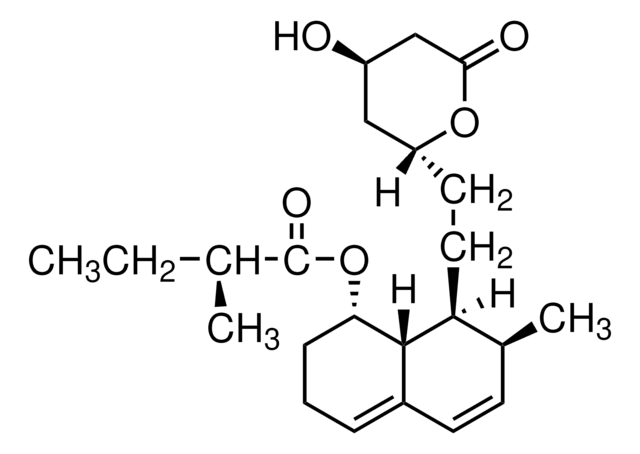
Fluvastatin sodium hydrate
≥98% (HPLC)
Synonym(e):
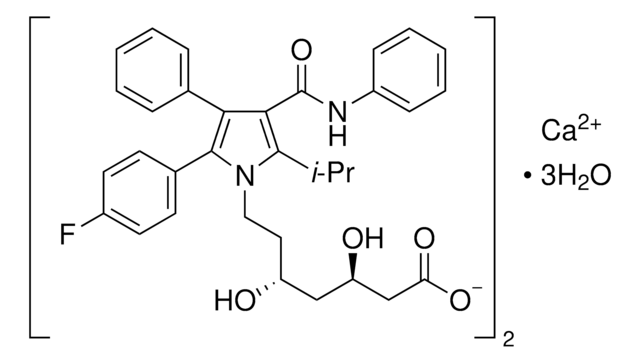
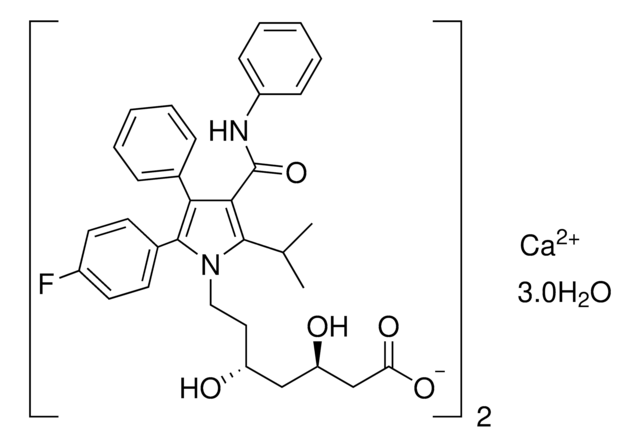
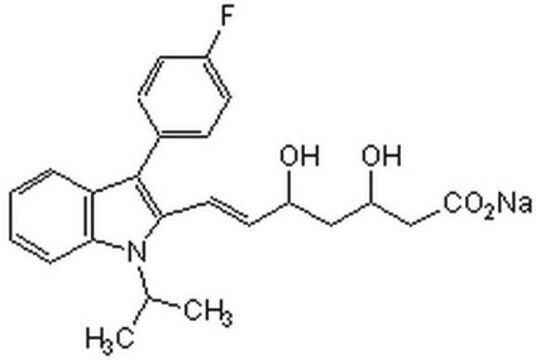
(±)-(3R*,5S*,6E)-7-[3-(4-Fluorophenyl)-1-(1-methyethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid sodium salt hydrate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Lagerbedingungen
desiccated
Farbe
white to tan
Löslichkeit
H2O: ≥9 mg/mL
Ersteller
Novartis
Lagertemp.
2-8°C
SMILES String
O.[Na+].CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)c(-c2ccc(F)cc2)c3ccccc13
InChI
1S/C24H26FNO4.Na.H2O/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);;1H2/q;+1;/p-1/b12-11+;;/t18-,19-;;/m1../s1
InChIKey
KKEMYLLTGGQWCE-PMRANXHDSA-M
Anwendung
- to examine its effect on β -glucan-induced training on immunity
- to investigate the effect of statins on the number of uncoupling protein 1 (UCP1)+ cells
- to determine its effect on insulin degrading enzyme (IDE) secretion from astrocytes
- to treat and study its effect on human umbilical vein endothelial cells (HUVECs) in vitro
- to test its anti-hepatitis C virus (HCV) activity
- as a cholesterol inhibitor
- to study its effects on β-glucan-induced monocyte immune training
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Verwandter Inhalt
Discover Bioactive Small Molecules for Lipid Signaling Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.