T7659
Trypsin Inhibitor, Defined (1X) Solution
Animal component free, BioReagent, suitable for cell culture
About This Item
Recommended Products
Quality Level
sterility
sterile-filtered
product line
BioReagent
form
solution
technique(s)
cell culture | mammalian: suitable
shipped in
dry ice
storage temp.
−20°C
Related Categories
Application
Physical form
Reconstitution
Not finding the right product?
Try our Product Selector Tool.
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
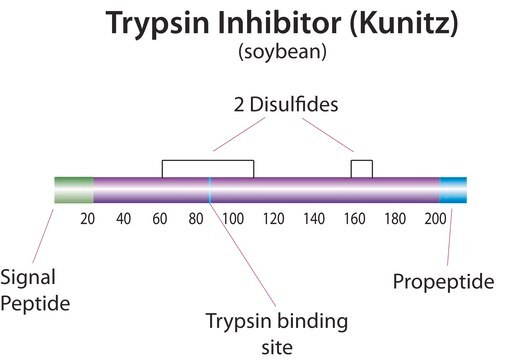
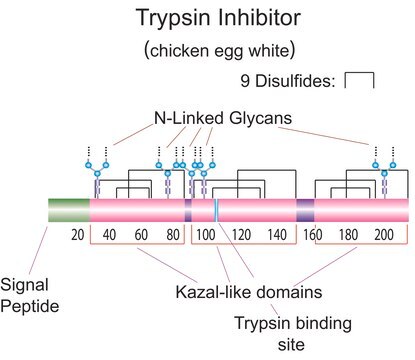
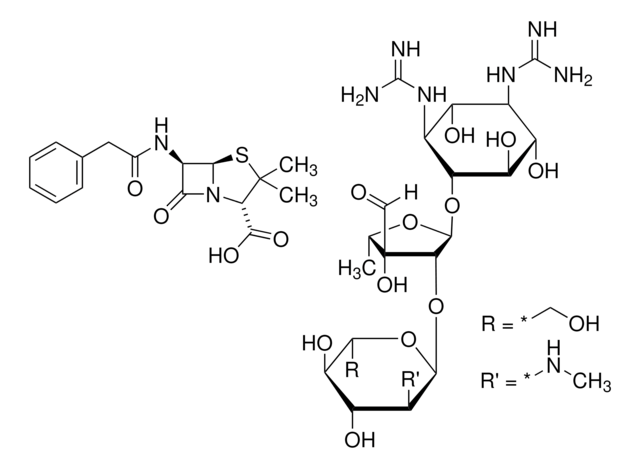
Natural trypsin Inhibitors also known as serine protease inhibitors (serpins) are the largest and most diverse family of protease inhibitors. Serpins control the activation and catabolism of proteins by the inhibition of serine proteases in vivo.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service