131377
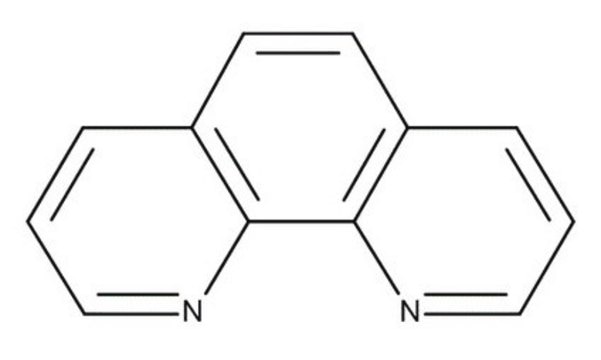
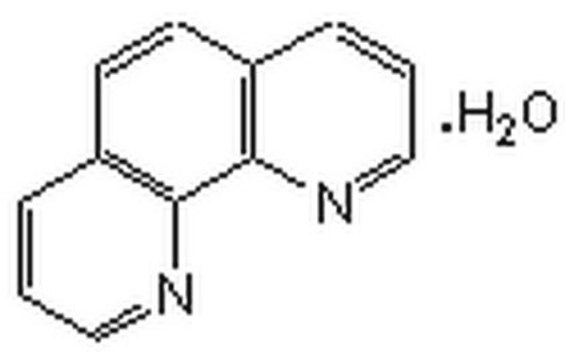
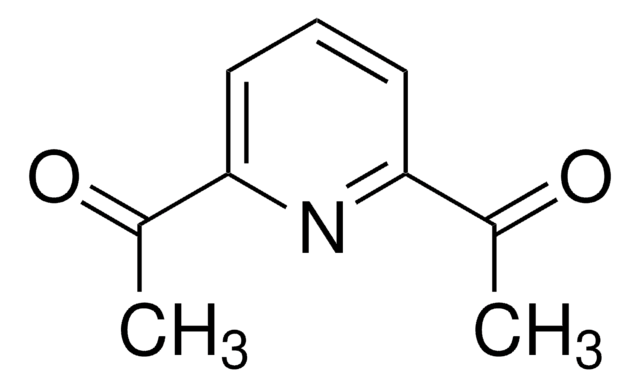
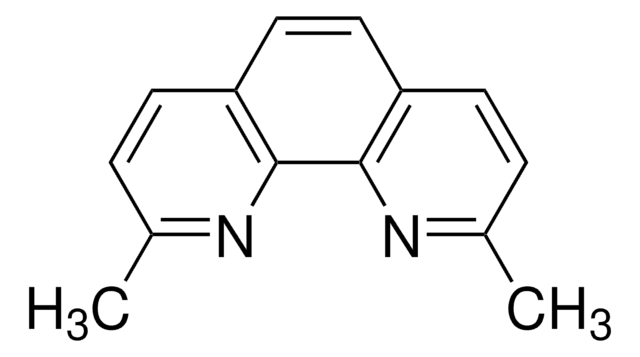
1,10-Phenanthroline
≥99%
Synonym(s):
o-phenanthroline
About This Item
Recommended Products
Quality Level
assay
≥99%
form
powder
mp
114-117 °C (lit.)
SMILES string
c1cnc2c(c1)ccc3cccnc23
InChI
1S/C12H8N2/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1/h1-8H
InChI key
DGEZNRSVGBDHLK-UHFFFAOYSA-N
Gene Information
human ... FNTA(2339)
Related Categories
Application
- A cathode buffer layer to improve the efficiency of organic solar cells.
- A conventional chelator to study its efficacy in Fenton′s reaction-luminol chemiluminescence system.
- A ligand in mild, copper (II)-catalyzed cross-coupling of organoboronic acids and sulfinate salts, leading to aryl- and alkenylsulfones.
- A versatile ligand employed in the spectrophotometric determination of metals and photocatalytic reduction of carbon dioxide.
- A building block for metallomacrocycles.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service