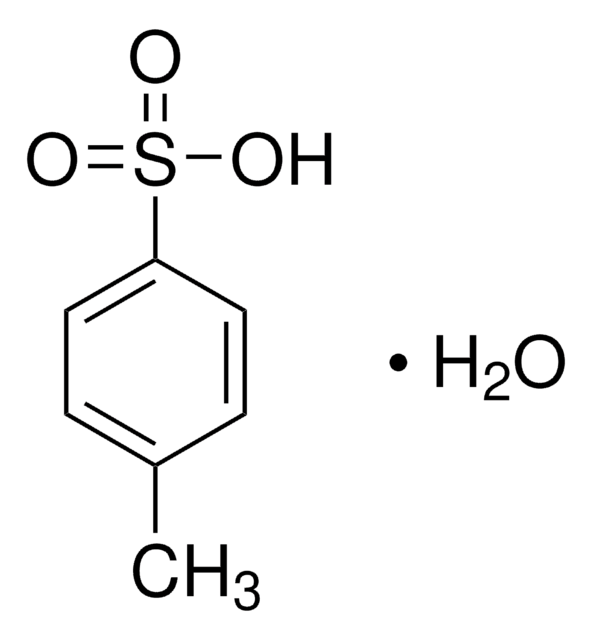
402885
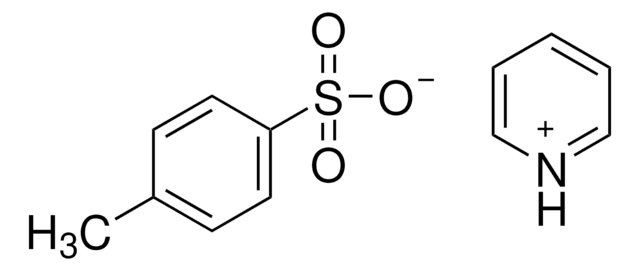
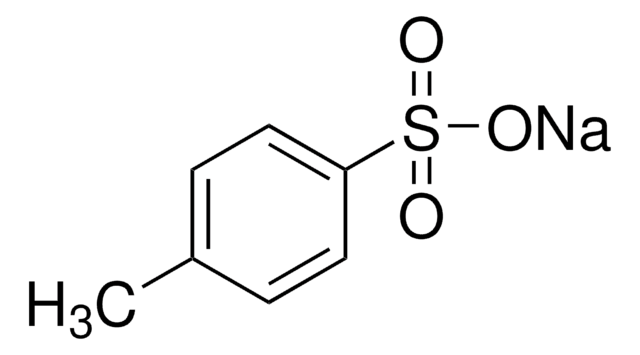
p-Toluenesulfonic acid monohydrate
ACS reagent, ≥98.5%
Synonym(s):
4-Methylbenzenesulfonic acid monohydrate, 4-Toluenesulfonic acid monohydrate, PTSA monohydrate, TsOH monohydrate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
vapor density
5.9 (vs air)
assay
≥98.5%
form
solid
clarity of soln
passes test
impurities
9.5-11.5% water
ign. residue
≤0.1%
mp
103-106 °C (lit.)
solubility
H2O: 10g + 50 mL, clear, colorless
anion traces
sulfate (SO42-): ≤0.3%
cation traces
Fe: ≤0.01%
Na: ≤0.002%
heavy metals (as Pb): ≤0.001%
SMILES string
[H]O[H].Cc1ccc(cc1)S(O)(=O)=O
InChI
1S/C7H8O3S.H2O/c1-6-2-4-7(5-3-6)11(8,9)10;/h2-5H,1H3,(H,8,9,10);1H2
InChI key
KJIFKLIQANRMOU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Unsymmetrical benzils.
- Highly substituted piperidines.
- 1,3,5-Trisubstituted benzenes by trimerization of alkynes.
- Triazoloquinazolinone and benzimidazoquinazolinone derivatives.
- 1,3,5-Trisubstituted pyrazoles derivatives.
- Selenated ketene dithioacetals.
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1C - STOT SE 3
target_organs
Respiratory system
Storage Class
8A - Combustible, corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service