1250008
USP
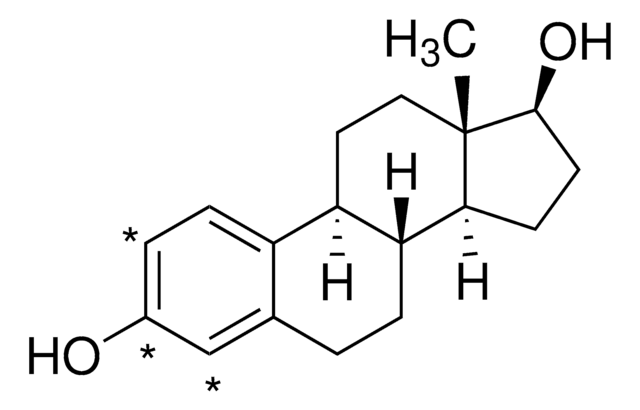
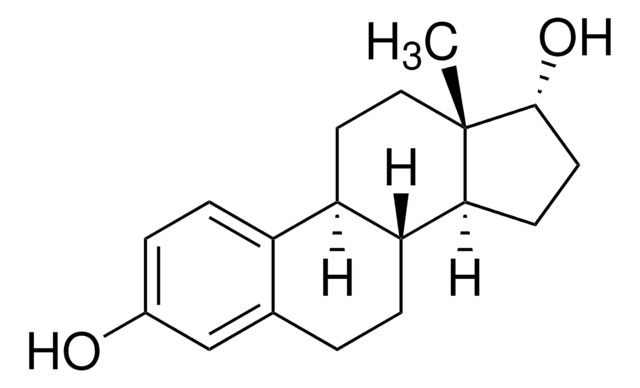
Estradiol
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
β-Estradiol, 1,3,5-Estratriene-3,17β-diol, 17β-Estradiol, 3,17β-Dihydroxy-1,3,5(10)-estratriene, Dihydrofolliculin
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
estradiol
manufacturer/tradename
USP
mp
176-180 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
O[C@H]1CC[C@@]2([H])[C@]3([H])CCC4=CC(O)=CC=C4[C@@]3([H])CC[C@@]21C
InChI
1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1
InChI key
VOXZDWNPVJITMN-ZBRFXRBCSA-N
Gene Information
human ... ESR1(2099)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Dehydroepiandrosterone with a high-fat diet treatment at inducing polycystic ovary syndrome in rat model: This study explores the impact of estradiol in a model of polycystic ovary syndrome, providing insights into hormone interactions and their implications in reproductive health (He et al., 2024).
- Ethanolic Extract of Xylopia aethiopica Attenuated Aluminum-Induced Ovarian Toxicity in Adult Female Wistar Rats: Research highlights the potential of estradiol-related compounds in mitigating heavy metal-induced ovarian damage, suggesting a protective role of estrogen receptor agonists in reproductive toxicology (Japhet et al., 2024).
- Connecting Bisphenol A Exposure to PCOS: Findings from a Case-Control Investigation: This study investigates the effects of environmental estrogens, mimicking estradiol, on the prevalence of polycystic ovary syndrome, emphasizing the importance of estrogen receptor interactions in environmental health studies (Patel et al., 2024).
- Combined collection systems of sewage and rainfall runoff seriously affect the spatial distributions of natural estrogens and their conjugates in river water: Insights from this study on the distribution of estrogenic compounds like estradiol in aquatic environments highlight significant implications for water quality and public health (Wu et al., 2024).
- Sex differences in the variability of fasting metabolism: This investigation into metabolic rate variations examines the role of estradiol in differing metabolic responses between sexes, contributing to a deeper understanding of hormonal impact on metabolic health (Bradshaw et al., 2024).
Biochem/physiol Actions
Analysis Note
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Lact. - Repr. 1A
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Estriol 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) 17-sulfate dipotassium salt; Estriol 3-sulfate sodium salt; β-Estradiol 3,17-disulfate dipotassium salt, ≥95%; β-Estradiol 17-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) sodium salt; Estrone 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-sulfate sodium salt, ≥93%; Estriol, ≥97%; Estrone 3-sulfate sodium salt, contains ~35% Tris as stabilizer; β-Estradiol, ≥98%; α-Estradiol, powder, ≥98% (TLC); Estrone, ≥99%
Protocols
Related Content
The Titan C18 column provided efficient and rapid resolution of thirteen related estrogenic compounds. Ultra Ultra high purity solvents provided robust operation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service