436674
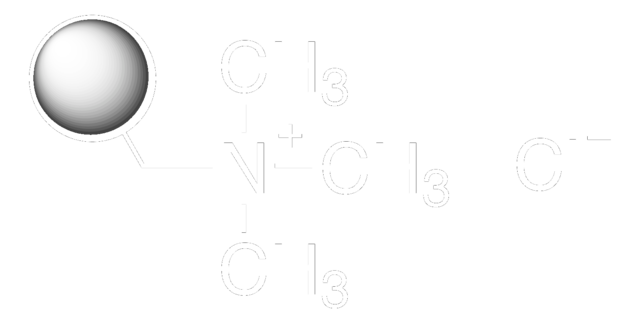
Amberlite™ FPA66 Ion Exchange Resin
free base, 16-50 mesh, anionic
Synonym(s):
strong cation exchange resin
About This Item
Recommended Products
product name
AmberLite™ FPA66 Anion Exchange Resin free base,
form
beads
Quality Level
parameter
60 °C OH- form max. temp.
moisture
40-46%
technique(s)
LPLC: suitable
matrix
styrene-divinylbenzene (macroporous)
matrix active group
polyamine functional group
particle size
300-1200 μm
50-125 mesh
operating pH
0-7
capacity
1.35 meq/mL by wetted bed volume
separation technique
anion exchange
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Specifications:
Application
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service