810173P
Avanti
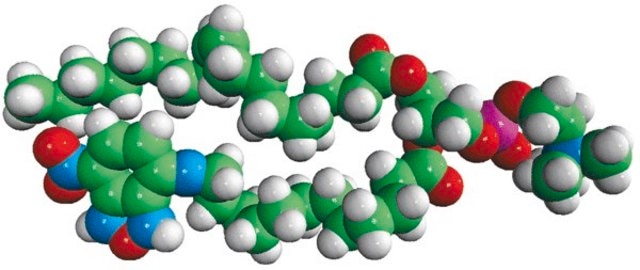
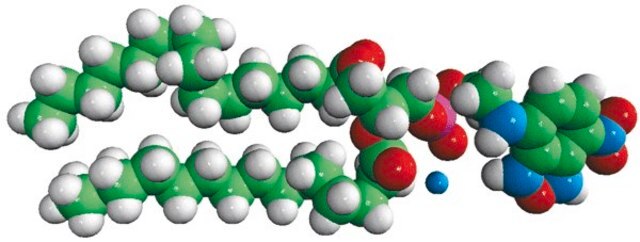
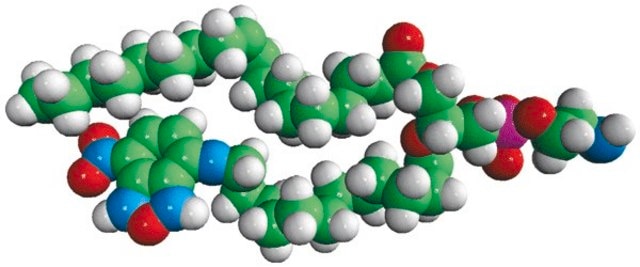
16:0-06:0 NBD PA
Avanti Polar Lipids 810173P, powder
Synonym(s):
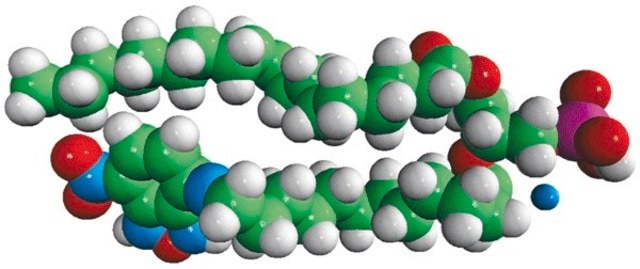
1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphate (ammonium salt)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C31H54N5O11P
CAS Number:
Molecular Weight:
703.76
UNSPSC Code:
12352211
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (810173P-1mg)
manufacturer/tradename
Avanti Polar Lipids 810173P
shipped in
dry ice
storage temp.
−20°C
General description
Phosphatidic acid (PA) is a simple anionic membrane phospholipid. The phospholipid contains phosphomonoester polar head group and two long hydrophobic acyl chains. 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) PA is a fluorescent analog with 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) attached to the sn-2 fatty acid of PA.
Application
16:0-06:0 NBD PA 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphate is suitable to measure the substrate transport by an increase in cellular fluorescence to determine type 4 P-type ATPases (P4-ATPase) discrimination and its glycerophospholipid (GPL) substrate selection through the directed evolution of a sphingomyelin (SM) flippase.
Biochem/physiol Actions
Phosphatidic acid (PA) is crucial for intracellular signaling involved various cellular processes including membrane trafficking and sperm hyper-activation. It plays a vital role in lipid synthesis.
Packaging
5 mL Amber Glass Screw Cap Vial (810173P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Phosphatidic acid binding proteins display differential binding as a function of membrane curvature stress and chemical properties
Putta P, et al.
Biochimica et Biophysica Acta, 1858(11), 2709-2716 (2016)
Phosphatidic acid in membrane rearrangements
Zhukovsky MA, et al.
Febs Letters, 593(17), 2428-2451 (2019)
Mikhail A Zhukovsky et al.
FEBS letters, 593(17), 2428-2451 (2019-08-01)
Phosphatidic acid (PA) is the simplest cellular glycerophospholipid characterized by unique biophysical properties: a small headgroup; negative charge; and a phosphomonoester group. Upon interaction with lysine or arginine, PA charge increases from -1 to -2 and this change stabilizes protein-lipid
A Chauhan et al.
Neurochemical research, 25(3), 423-429 (2000-04-13)
Fibrillar amyloid beta-protein (Abeta) is the major protein of amyloid plaques in the brains of patients with Alzheimer's disease (AD). The mechanism by which normally produced soluble Abeta gets fibrillized in AD is not clear. We studied the effect of
Karen M Henkels et al.
Oncotarget, 7(30), 47002-47017 (2016-06-04)
The intracellular concentration of the mitogen phosphatidic acid (PA) must be maintained at low levels until the need arises for cell proliferation. How temporal and spatial trafficking of PA affects its target proteins in the different cellular compartments is not
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service