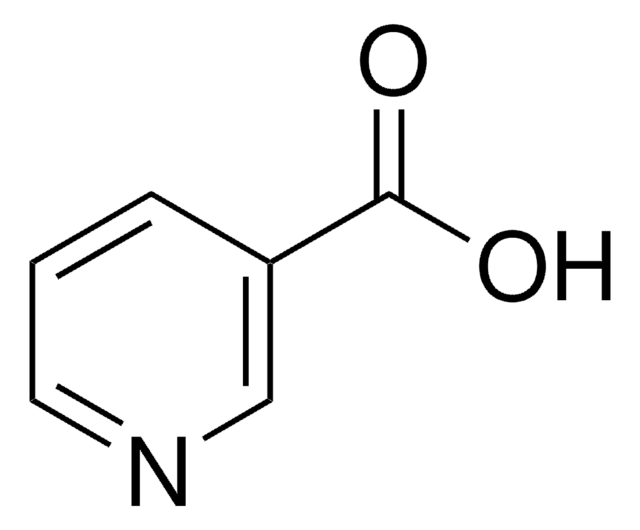
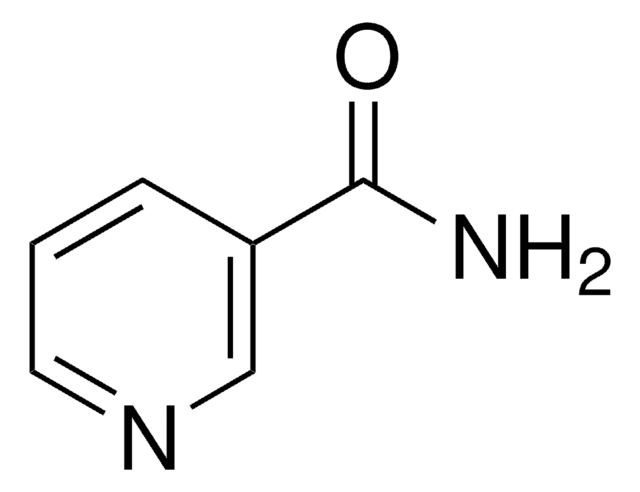
I17451
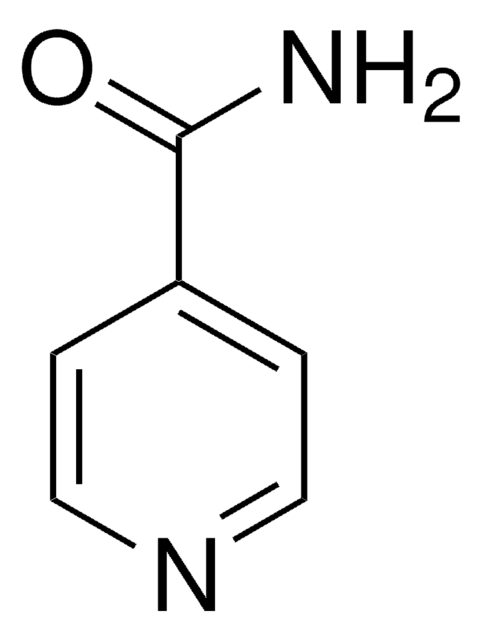
Isonicotinamide
ReagentPlus®, 99%
Synonym(s):
Pyridine-4-carboxylic acid amide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
Beilstein:
2173
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
mp
155-157 °C (lit.)
SMILES string
NC(=O)c1ccncc1
InChI
1S/C6H6N2O/c7-6(9)5-1-3-8-4-2-5/h1-4H,(H2,7,9)
InChI key
VFQXVTODMYMSMJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Isonicotinamide (pyridine-4-carboxamide) can be used as a heterocyclic building block to synthesize:
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
- 4-oxo-1,3-thiazinan-3-yl isonicotinamide derivatives as potential anti-tubercular agents.
- Organotin(IV) complexes of isonicotinamide via synthesis of phosphoramidate ligands for various biological activity studies.
- Bis-pyridinium isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as potent reactivators sarin.
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gareth Arnott et al.
Organic letters, 10(14), 3089-3092 (2008-06-17)
Treatment of N-arylisonicotinamides with trifluoromethanesulfonic anhydride triggers intramolecular nucleophilic attack of the aryl ring on the 4-position of the pyridinium intermediate. The products are spirocyclic dihydropyridines which can be converted to valuable spirocyclic piperidines related to biologically active molecules such
Rodrigo A de Souza et al.
European journal of medicinal chemistry, 45(11), 4863-4868 (2010-08-21)
Complexes of the type trans-[PdX(2)(isn)(2)] {X = Cl (1), N(3) (2), SCN (3), NCO (4); isn = isonicotinamide} were synthesized and evaluated for in vitro antimycobacterial and antitumor activities. The coordination mode of the isonicotinamide and the pseudohalide ligands was
Jemma Senczyszyn et al.
Organic letters, 15(8), 1922-1925 (2013-04-04)
On treatment with acylating or sulfonylating agents, N-alkenyl pyridine carboxamides (N-pyridinecarbonyl enamines) undergo a dearomatizing cyclization initiated by pyridine acylation and followed by intramolecular trapping of the resulting pyridinium cation. The products are spirocyclic dihydropyridines which may be further elaborated
Sarah Boyd et al.
Journal of pharmaceutical sciences, 99(9), 3779-3786 (2010-07-29)
The solubility and crystal growth of the 1:1 cocrystal between benzoic acid and isonicotinamide from 95% ethanol was studied through the creation of a ternary phase diagram at differing temperatures and turbidity measurements. From the solubility measurements thermodynamic properties of
Shaun R Stauffer et al.
Bioorganic & medicinal chemistry letters, 17(6), 1788-1792 (2007-01-30)
A series of low-molecular weight 2,6-diamino-isonicotinamide BACE-1 inhibitors containing an amine transition-state isostere were synthesized and shown to be highly potent in both enzymatic and cell-based assays. These inhibitors contain a trans-S,S-methyl cyclopropane P(3) which bind BACE-1 in a 10s-loop
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service