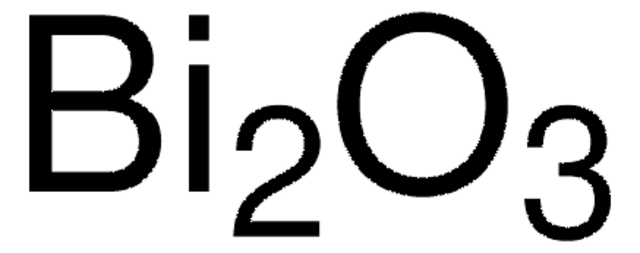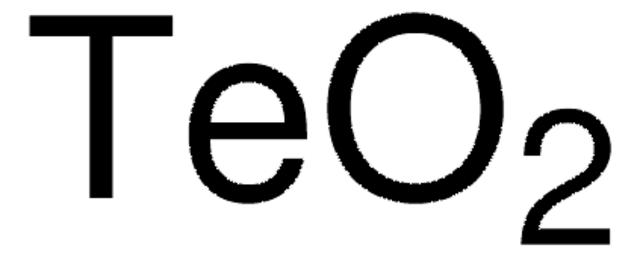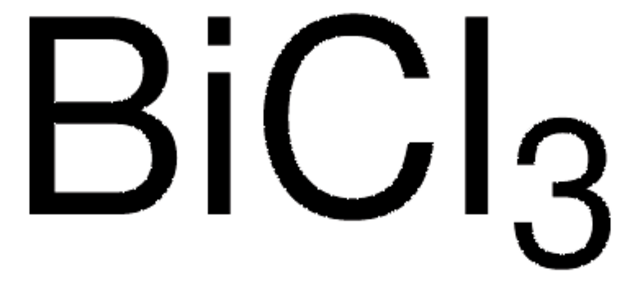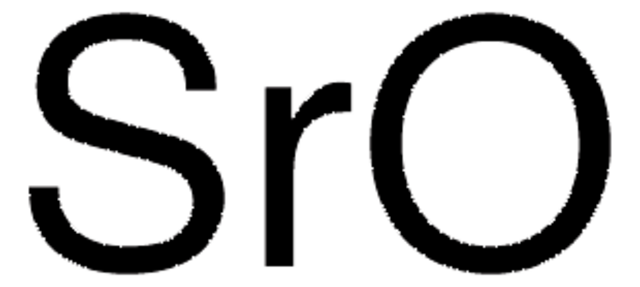95381
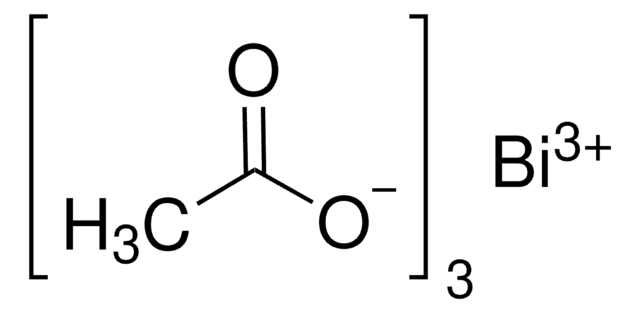
Bismut(III)-Oxid
purum, ≥98.0% (KT)
Synonym(e):
Dibismuttrioxid
About This Item
Empfohlene Produkte
Qualität
purum
Qualitätsniveau
Assay
≥98.0% (KT)
Form
crystals
Eignung der Reaktion
reagent type: catalyst
core: bismuth
Verlust
≤0.2% loss on ignition
SMILES String
O=[Bi]O[Bi]=O
InChI
1S/2Bi.3O
InChIKey
WMWLMWRWZQELOS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Leistungsmerkmale und Vorteile
WGK
nwg
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.