133302
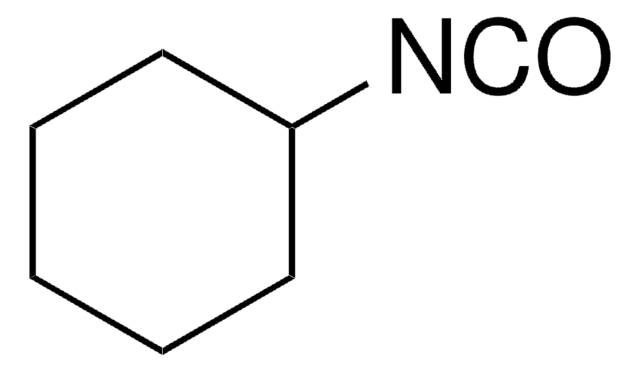
Cyclohexylisocyanid
98%
Synonym(e):
Isocyancyclohexan
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
liquid
Brechungsindex
n20/D 1.45 (lit.)
Dichte
0.878 g/mL at 25 °C (lit.)
Lagertemp.
2-8°C
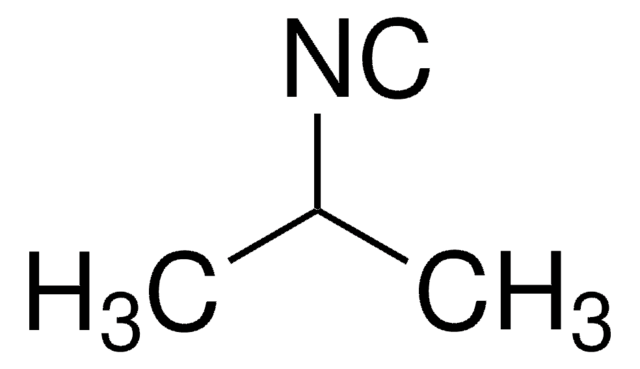
SMILES String
[C-]#[N+]C1CCCCC1
InChI
1S/C7H11N/c1-8-7-5-3-2-4-6-7/h7H,2-6H2
InChIKey
XYZMOVWWVXBHDP-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Isocyanide in coordination chemistry: The study on mixed "2 + 1" tricarbonyl dithiocarbamate complexes highlights Cyclohexyl isocyanide′s role as an effective monodentate ligand, contributing to advancements in radiopharmaceutical applications using Re, Tc, and Re isotopes (Shegani et al., 2021).
- Organic synthesis reagent in vascular treatments: The article discusses the use of Cyclohexyl isocyanide in modifying the endocannabinoid system, emphasizing its potential in developing treatments for conditions like hypertension through biochemical pathway modulation (Baranowska-Kuczko et al., 2021).
- Chemical process optimization in dye decolorization: Cyclohexyl isocyanide plays a crucial role in the covalent immobilization of enzymes used for the decolorization of textile dyes, demonstrating its utility in environmental chemistry and industrial applications related to pollution control (Salami et al., 2018).
- Application in nanocellulose modification: Demonstrates the versatility of Cyclohexyl isocyanide in nanotechnology by facilitating the covalent attachment of temperature-responsive polymers to cellulose nanofibrils, enhancing the material′s properties for use in smart textiles and responsive materials (Khine et al., 2018).
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Lagerklassenschlüssel
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
170.6 °F - closed cup
Flammpunkt (°C)
77 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.