1494909
USP
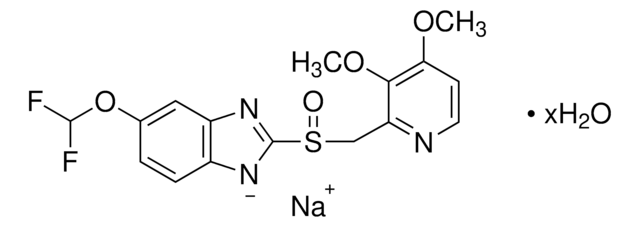
Pantoprazole Related Compound A
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
Pantoprazole sulfone, 5-(Difluoromethoxy)-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfone}-1H-benzimidazole
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
pantoprazole
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
InChI
1S/C16H15F2N3O5S/c1-24-13-5-6-19-12(14(13)25-2)8-27(22,23)16-20-10-4-3-9(26-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)
InChI key
FCJYMBZQIJDMMM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Transcriptional profiling for drug repurposing in glioblastoma: This study utilized transcriptional profiling to identify therapeutic targets and repurpose drugs, including pantoprazole-related compounds, for treatment in glioblastoma. This approach underscores the utility of pantoprazole impurities in exploring new therapeutic avenues in pharmaceutical reference material research (Roddy et al., 2023).
- Gastroprotective agent utilization: A drug utilization study in medicine and surgery wards highlighted the significant role of pantoprazole and its related compounds in managing gastrointestinal protection. This research emphasizes the importance of pantoprazole as a proton pump inhibitor and its impurities in ensuring the efficacy and safety of gastroprotective therapies (Koyani et al., 2023).
- Synthesis and analysis of pantoprazole sodium sesquihydrate-related compound E: This study explored the synthesis and mechanism of formation of a specific pantoprazole-related compound, offering insights into the chemical stability and quality control of pantoprazole as a pharmaceutical ingredient. Such research is crucial for enhancing analytical methods in pharmaceutical research and ensuring drug safety and efficacy (Yari et al., 2019).
Analysis Note
Other Notes
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service