1271700
USP
Fluconazole
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
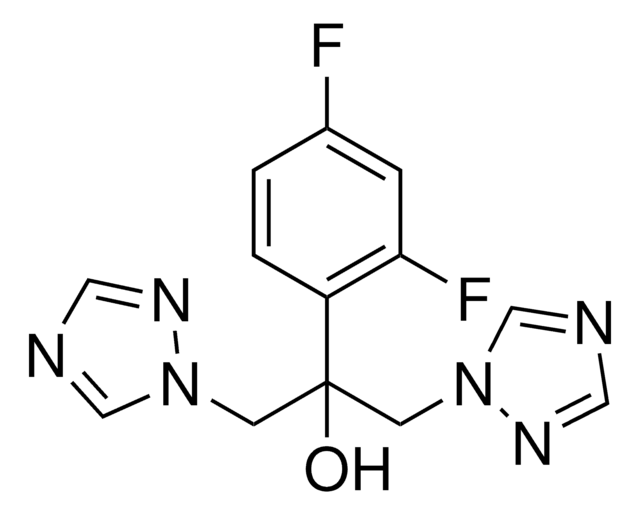
Fluconazole, 2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
fluconazole
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
FC1=CC(F)=C(C(CN2N=CN=C2)(O)CN3N=CN=C3)C=C1
InChI
1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2
InChI key
RFHAOTPXVQNOHP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Enhanced antifungal activity of fluconazole: A study optimized fluconazole-embedded transfersomal gel, demonstrating improved antifungal activity and compatibility. This research is crucial for enhancing fluconazole′s efficacy against resistant fungal strains, making it a pivotal tool in the fight against fungal infections (Cheng et al., 2024).
- Antifungal mechanisms against drug-resistant strains: Research on the antifungal activity of a trypsin inhibitor from chia seeds against fluconazole-resistant Candida species assessed its potential as a novel therapeutic approach. This study contributes valuable insights into natural compounds enhancing fluconazole′s effectiveness, crucial for developing alternative antifungal therapies (Nogueira et al., 2024).
- Advancements in fungal pathogenesis: The isolation and identification of Wickerhamiella tropicalis from blood culture using MALDI-MS highlight innovative diagnostic techniques that enhance the understanding of fungal pathogenesis. This research is essential for advancing microbial diagnostics and tailoring treatments to combat invasive fungal infections effectively (Takei et al., 2024).
Biochem/physiol Actions
Analysis Note
Other Notes
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Lact. - Repr. 1B
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service