SMB00607
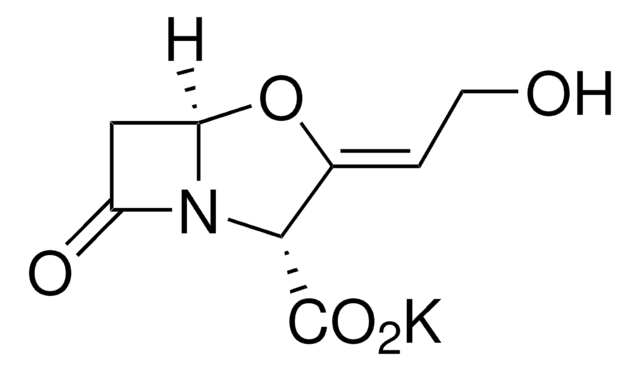
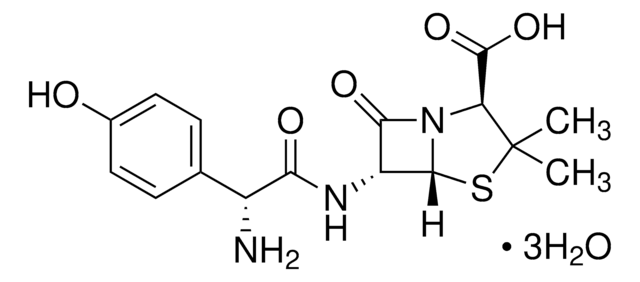
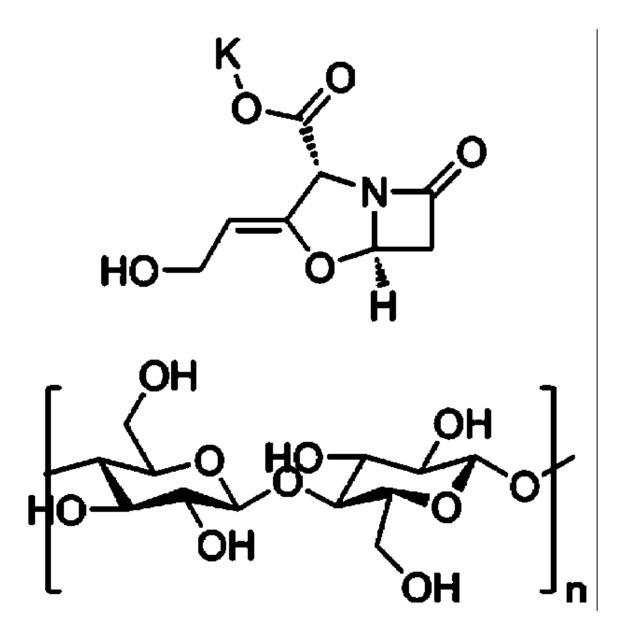
Amoxicillin trihydrate: potassium clavulanate (4:1)
Synonym(s):
Amoxicillin Trihydrate: Potassium Clavulanate
About This Item
Recommended Products
form
powder
Quality Level
storage condition
(Keep container tightly closed in a dry and well-ventilated place. Never allow produc to get in contact with water during storage.)
color
white to yellow
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
Related Categories
General description
Application
Biochem/physiol Actions
Clavulanate is a β-lactam antibiotic related to the penicillins. Clavulanate competitively and irreversibly inhibits a wide variety of β-lactamases found in bacteria that are resistant to penicillins and cephalosporins.
Formulations of amoxicillin with clavulanic acid prevent the degradation of amoxicillin by β-lactamase enzymes. This increases amoxicillin′s antibacterial activity against bacteria normally resistant to β-lactam antibiotics.
Packaging
Components
Storage and Stability
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Flam. Sol. 2 - Resp. Sens. 1 - Self-heat. 2 - Skin Sens. 1
supp_hazards
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service