P6516
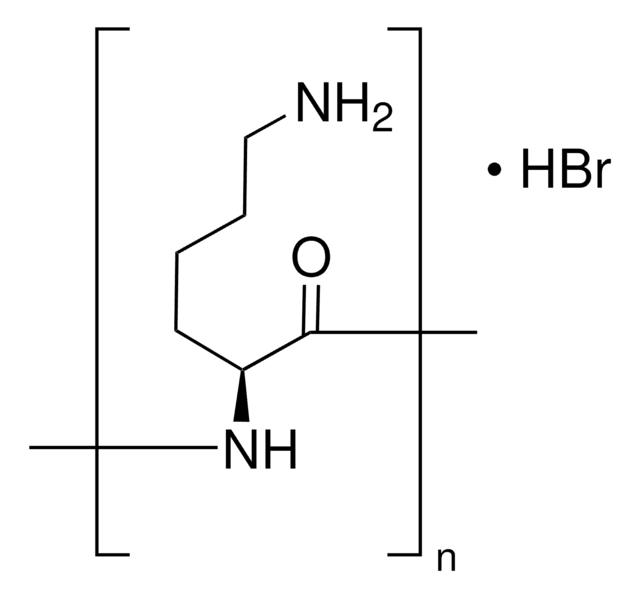
Poly-L-lysine hydrobromide
suitable for cell culture, Mol wt ≤15,000 by MALLS
Synonym(s):
L-Lysine homopolymer hydrobromide
About This Item
Recommended Products
product name
Poly-L-lysine hydrobromide, mol wt 4,000-15,000 by viscosity
form
solid
Quality Level
mol wt
≤15,000 by MALLS
4,000-15,000 by viscosity
greener alternative product score
old score: 51
new score: 43
Find out more about DOZN™ Scoring
greener alternative product characteristics
Designing Safer Chemicals
Safer Solvents and Auxiliaries
Reduce Derivatives
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
technique(s)
cell culture | mammalian: suitable
color
white to off-white
application(s)
cell analysis
greener alternative category
storage temp.
−20°C
SMILES string
Cl.NCCCCC(N)C(O)=O
InChI
1S/C18H38N6O4/c19-10-4-1-7-13(22)16(25)23-14(8-2-5-11-20)17(26)24-15(18(27)28)9-3-6-12-21/h13-15H,1-12,19-22H2,(H,23,25)(H,24,26)(H,27,28)/t13-,14-,15-/m0/s1
InChI key
WBSCNDJQPKSPII-KKUMJFAQSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a component of polyplexes and in DNA condensation experiments
- for coating circular mica disks for atomic force microscopy studies and SuperFrost glass slides for immunofluorescence studies of in HL-60 cells
- as a reference standard for generating calibration curve for the quantification of ε- Poly-L-lysine
Biochem/physiol Actions
Components
Caution
Preparation Note
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Kanjiro Miyata (The University of Tokyo, Japan) provides insights on the rational design of polymeric materials for “smart” oligonucleotide delivery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service