G8795
Anti-GAPDH antibody, Mouse monoclonal
clone GAPDH-71.1, purified from hybridoma cell culture
Synonym(s):
GAPDH Antibody - Monoclonal Anti-GAPDH antibody produced in mouse, Gapdh Antibody, Anti-G3PD, Anti-G3PDH, Anti-Glyceraldehyde-3-phosphate dehydrogenase, Loading Control
About This Item
Recommended Products
biological source
mouse
Quality Level
conjugate
unconjugated
antibody form
purified from hybridoma cell culture
purified immunoglobulin
antibody product type
primary antibodies
clone
GAPDH-71.1, monoclonal
form
buffered aqueous solution
mol wt
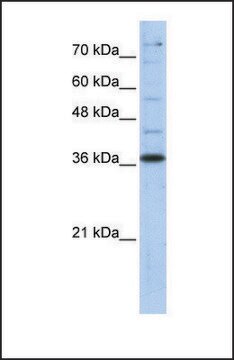
antigen ~37 kDa
species reactivity
bovine, turkey, canine, chicken, monkey, mink, mouse, human, rabbit, rat, hamster
should not react with
prokaryotes
packaging
antibody small pack of 25 μL
concentration
~1 mg/mL
technique(s)
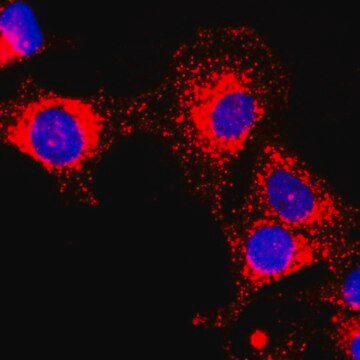
immunocytochemistry: suitable
indirect ELISA: suitable
microarray: suitable
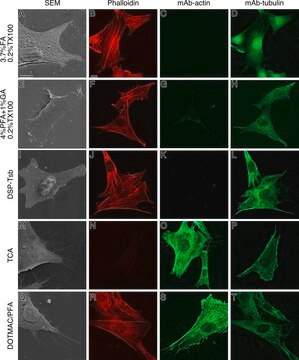
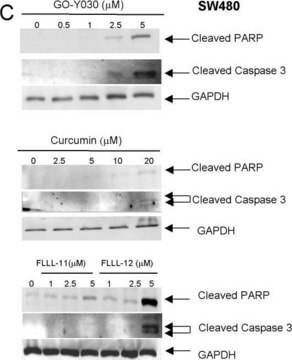
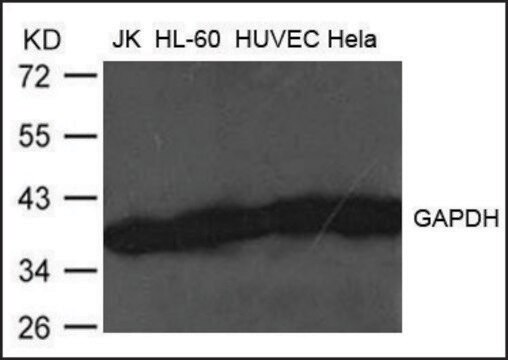
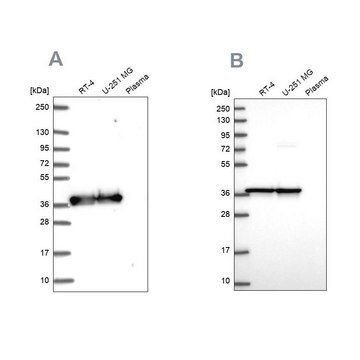
western blot: 0.025-0.05 μg/mL using A431 total cell extract
isotype
IgM
UniProt accession no.
application(s)
research pathology
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... GAPDH(2597)
mouse ... Gapdh(14433)
rat ... Gapdh(24383)
General description
Specificity
Immunogen
Application
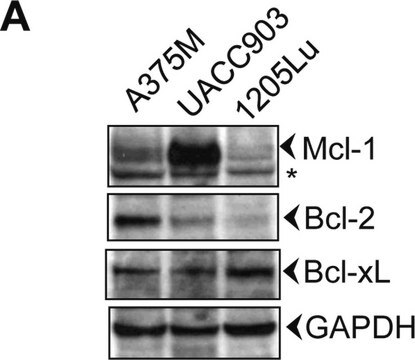
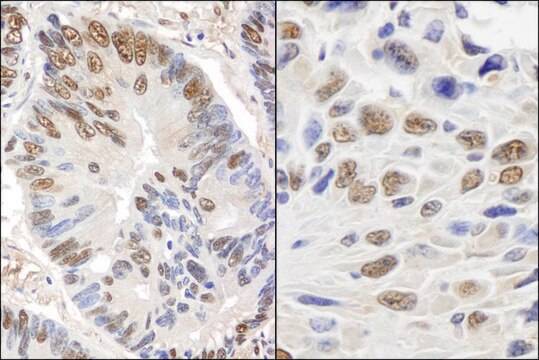
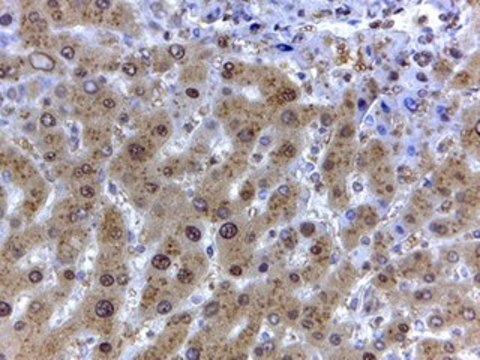
- protein extracted from heart tissue of mice at a working dilution of 1:25,000
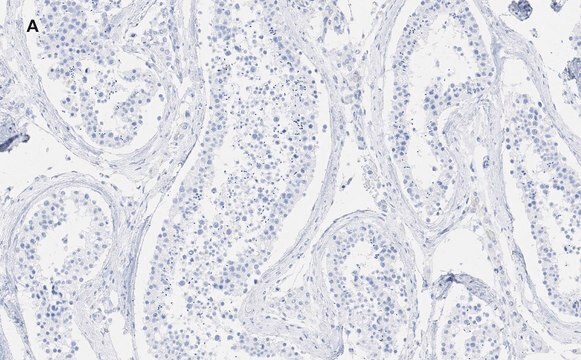
- myelin and axogliasomal fractions from human CNS
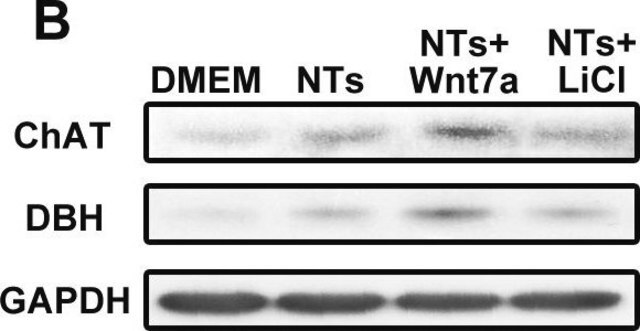
- nuclear and cytoplasmic fractions from TBP-13Q and TBP-105Q PC12 cells following recovery from heat shock
- protein from bovine immortalized luteal endothelial cells
- renal tubular epithelial cell extract
- proteins from mouse embryonic fibroblasts
- protein extract from ventricular myocardium tissues
- A431 total cell extract at a working concentration of 0.025-0.05μg/mL
Biochem/physiol Actions
Physical form
Storage and Stability
For extended storage, freeze in working aliquots. Repeated freezing and thawing, or storage in “frostfree” freezers, is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use. Working dilution samples should be discarded if not used within 12 hours.
Disclaimer
Still not finding the right product?
Give our Product Selector Tool a try.
recommended
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
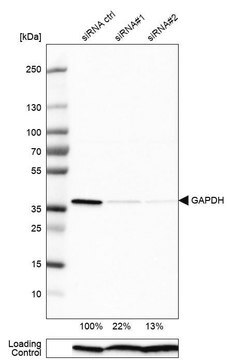
Loading controls in western blotting application.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service