General description
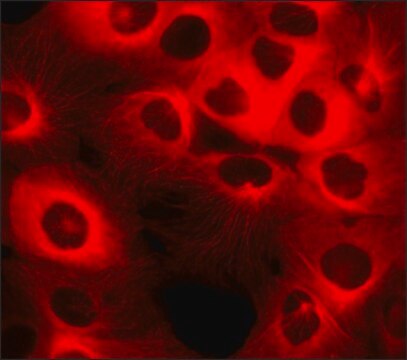
MCF10A CELLS HER2 (-/-) are pre-neoplastic mammary epithelial cells from a human adult female with a ZFN knock out modification.
This cell line corresponds to ATCC Catalog No. CRL-10317.
Application
Cell freezing medium-DMSO 1X, Cat. No. C6164
Media renewal changes two to three times per week.
Rapidly thaw vial by gentle agitation in 37°C water bath (~2 minutes), keeping vial cap out of the water. Decontaminate with 70% ethanol, add 9 mL culture media and centrifuge 125 x g (5-7 minutes). Resuspend in complete culture media and incubate at 37°
The base medium for this cell line is Dulbecco′s Modified Eagle′s Medium/Ham′s Nutrient Mixture F-12, Cat. No. 51448C. To make the complete growth medium, add the following components to the base medium: horse serum, Cat No. H1270, to a final concentration (v/v) of 5%; cholera toxin, Cat No. C8052, to a final concentration of 1 ng/mL; human insulin, Cat No. I9278, to a final concentration of 10 ug/mL; epidermal growth factor, Cat No. E9644, to a final concentration of 10 ng/mL; and hydrocortisone, Cat No. H6909, to a final concentration of 0.5 ug/mL.
Biochem/physiol Actions
Human epidermal growth factor receptor 2 (HER2) signaling pathway stimulates cell proliferation and survival in the majority of breast cancers. Thus, overexpression of this protein causes breast cancer. Heterodimeric complex of HER2 and phosphatidylinositide 3-kinase (PI3K) is the most potent stimulator of the PI3K/AKT anti-apoptosis pathway. Increased expression of HER2 is also observed in ovarian, colorectal, pancreatic, endometrial and gastric cancers. Trastuzumab, an antibody, works by binding to a domain in the external domain of HER2. This domain is missing in p95, a truncated form of HER2, and hence these cancer cells show resistance to trastuzumab. HER2 protein can be used as a prognostic marker and as a therapeutic option for gynecologic cancers.
Features and Benefits
These MCF10A cells are adherent with a doubling time of approx. 20 hours
Zinc finger nuclease (ZFN) knockout on chromosome 17q21.1
Quality
Tested for Mycoplasma, sterility, post-freeze viability, short terminal repeat (STR) analysis for cell line identification, cytochrome oxidase I (COI) analysis for cell line species confirmation.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.