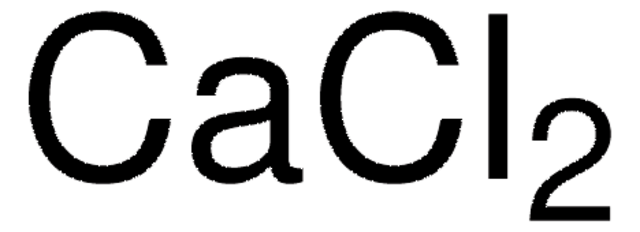43815
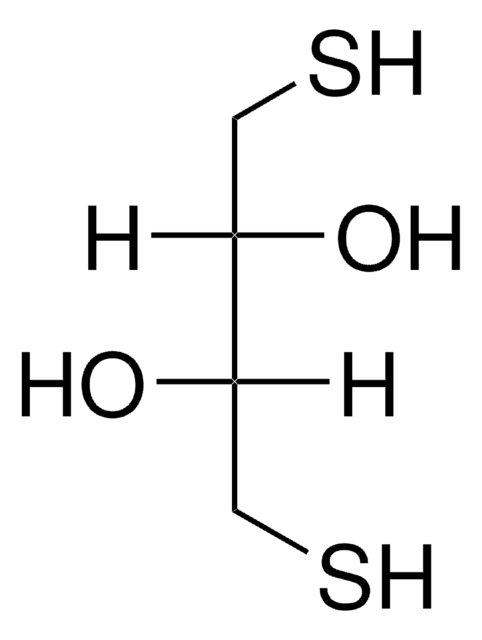
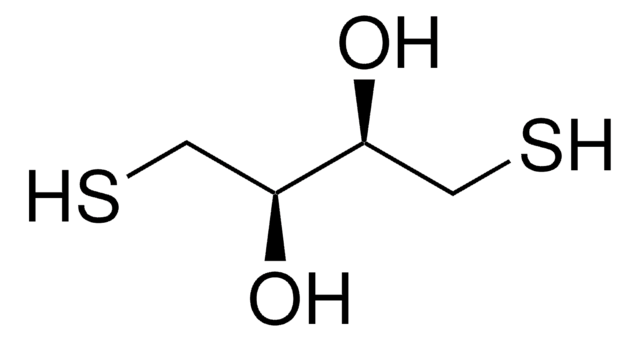
DL-Dithiothreitol
BioUltra, for molecular biology, ≥99.5% (RT)
Synonym(s):
(±)-Dithiothreitol, rac-Dithiothreitol, Dithiothreitol, threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT
About This Item
Recommended Products
grade
for molecular biology
Quality Level
product line
BioUltra
assay
≥99.5% (RT)
form
powder
reaction suitability
reagent type: reductant
impurities
DNases, none detected
RNases, none detected
insoluble matter, passes filter test
phosphatases, none detected
proteases, none detected
≤0.3% 4,5-dihydroxy-1,2-dithiane
pH
4.0-6.5 (25 °C, 0.1 M in H2O)
mp
41-44 °C (lit.)
solubility
H2O: 0.1 M at 20 °C, clear, colorless
anion traces
sulfate (SO42-): ≤50 mg/kg
cation traces
Al: ≤5 mg/kg
As: ≤0.5 mg/kg
Ba: ≤5 mg/kg
Bi: ≤5 mg/kg
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Li: ≤5 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Mo: ≤5 mg/kg
Na: ≤500 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Sr: ≤5 mg/kg
Zn: ≤5 mg/kg
λ
0.1 M in H2O
UV absorption
λ: 260 nm Amax: 0.400
λ: 280 nm Amax: 0.100
SMILES string
O[C@H](CS)[C@H](O)CS
storage temp.
2-8°C
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
VHJLVAABSRFDPM-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as an enzyme stabilizer to maintain exebacase stability
- for peptide extraction and protein denaturation
- in sample preparation and reversal of formaldehyde crosslinks before mass spectrometry
- as a buffer component to extract synaptogliosome
Biochem/physiol Actions
Features and Benefits
- BioUltra grade powder suitable for molecular biology
- DNase, RNase, phosphatase, and protease-free insoluble matter, passes the filter test
- Stringently tested and free from trace metals
Other Notes
comparable product
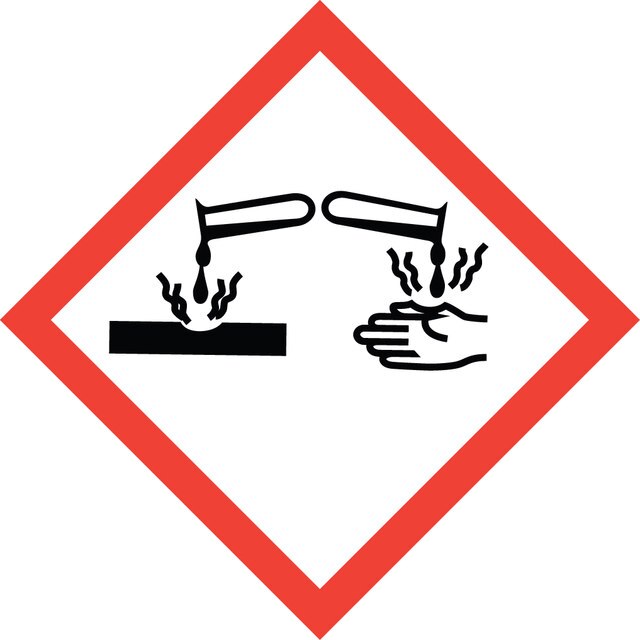
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Understand how mRNA vaccines induce immunity. and read how synthetic mRNA is prepared for vaccine immunogens and other biopharmaceuticals. Find reagents for synthesis of mRNA.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service