22620
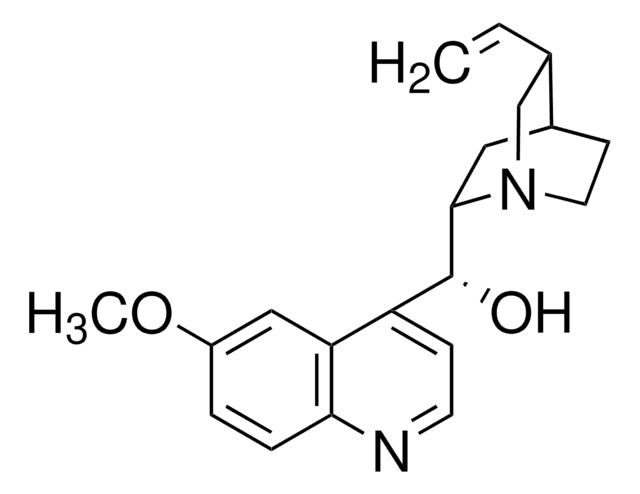
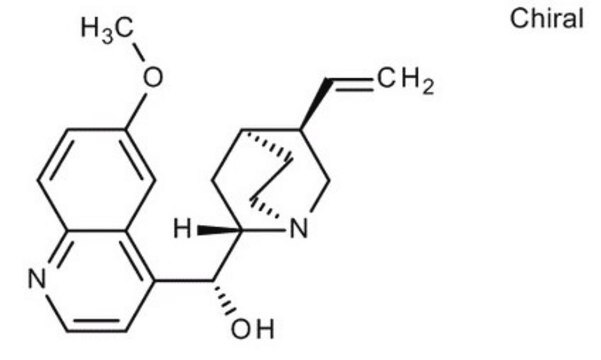
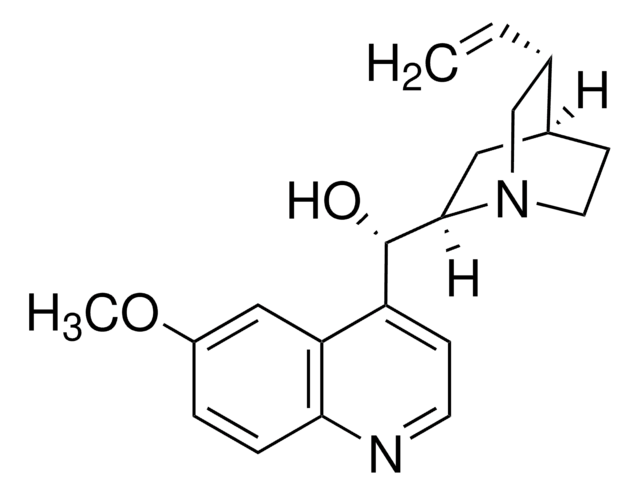
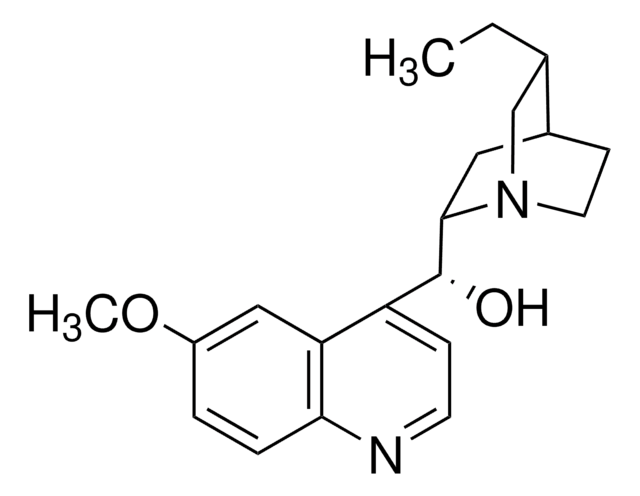
Quinine
suitable for fluorescence, anhydrous, ≥98.0% (dried material, NT)
Synonym(s):
6′-Methoxycinchonidine
About This Item
Recommended Products
Quality Level
assay
≥98.0% (dried material, NT)
form
powder
optical activity
[α]20/D −126±5°, c = 1% in chloroform
impurities
≤5% dihydroquinine (HPLC)
loss
≤1% loss on drying, 110 °C
mp
173-175 °C (lit.)
solubility
H2O: soluble
fluorescence
λex 347 nm; λem 448 nm in 0.5 M sulfuric acid
suitability
suitable for fluorescence
SMILES string
COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1
InChI key
LOUPRKONTZGTKE-WZBLMQSHSA-N
Gene Information
human ... ABCB1(5243) , CYP2C9(1559) , CYP2D6(1565)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To study its in vitro antimalarial activity in combination with omeprazole.
- To analyze its effect on viscosity and friction of saliva.
- As a test agent to study its impact on the accumulation of the fluorescent P-glycoprotein (Pgp) substrates in P-glycoprotein overexpressing breast cancer cells.
- To study its influence on the pyramidal cell intrinsic properties, extracellular potassium transients, and epileptiform activity in vitro.
- As a reference compound to identify alkaloids by phytochemical screening of Deianira erubescens, Strychnos pseudoquina and Remijia ferruginea plants.
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service