X3877
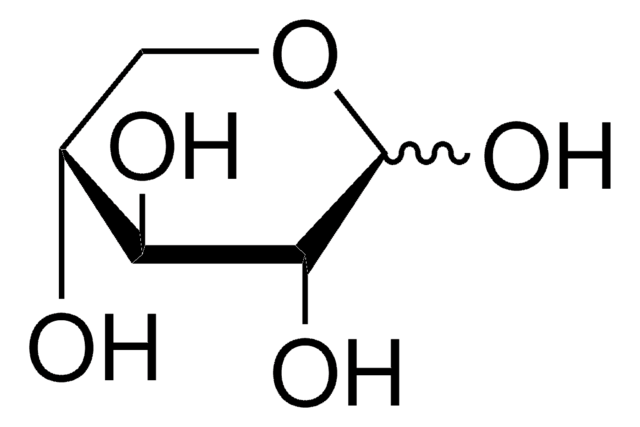
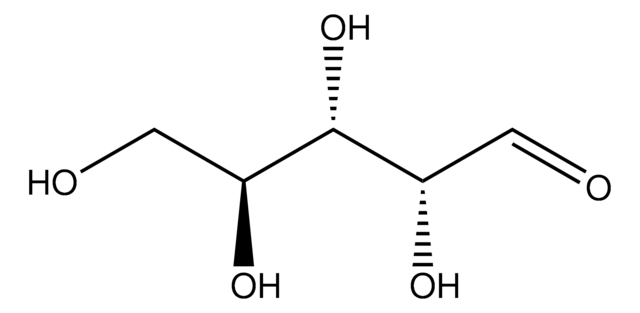
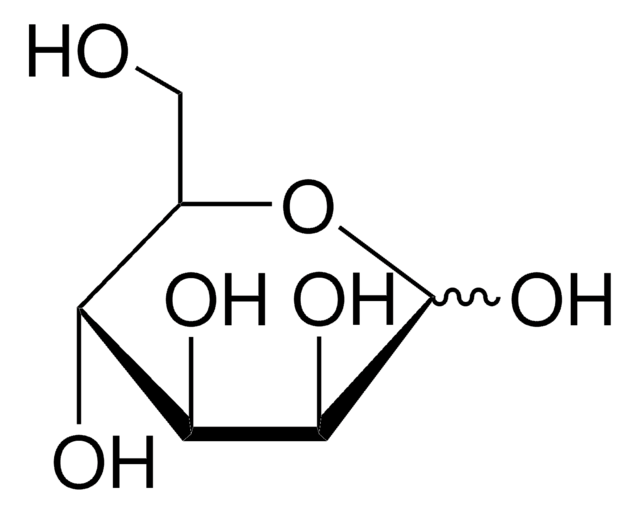
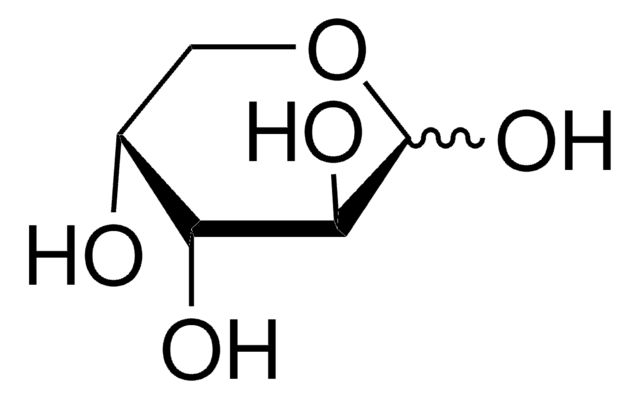
D-(+)-Xylose
BioXtra, ≥99% (GC)
Synonym(s):
D-xylopyranose
About This Item
Recommended Products
product line
BioXtra
Quality Level
assay
≥99% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
impurities
≤0.0005% Phosphorus (P)
≤0.1% Insoluble matter
ign. residue
≤0.1%
color
white
useful pH range
6.0-6.5 (20 °C, 100 g/L)
mp
154-158 °C (lit.)
solubility
H2O: 1 M, clear, colorless
anion traces
chloride (Cl-): ≤0.05%
sulfate (SO42-): ≤0.05%
cation traces
Al: ≤0.0005%
Ca: ≤0.0005%
Cu: ≤0.0005%
Fe: ≤0.0005%
K: ≤0.005%
Mg: ≤0.0005%
NH4+: ≤0.05%
Na: ≤0.005%
Pb: ≤0.001%
Zn: ≤0.0005%
SMILES string
O[C@@H]1COC(O)[C@H](O)[C@H]1O
InChI
1S/C5H10O5/c6-2-1-10-5(9)4(8)3(2)7/h2-9H,1H2/t2-,3+,4-,5?/m1/s1
InChI key
SRBFZHDQGSBBOR-IOVATXLUSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Structural and molecular insights into a bifunctional glycoside hydrolase 30 xylanase specific to glucuronoxylan: This study provides a detailed analysis of the enzyme′s structure and function, demonstrating D-(+)-Xylose′s role in enhancing our understanding of xylose metabolism in industrial applications such as biofuel production and biotechnological innovations (Pentari et al., 2024).
- Efficient production of 1,2,4-butanetriol from corn cob hydrolysate by metabolically engineered Escherichia coli: Highlights the utilization of D-(+)-Xylose from agricultural waste, optimizing processes for sustainable biofuel production, emphasizing the sugar′s pivotal role in renewable energy research (Li et al., 2024).
- Biochemical characterization of a xylose-tolerant GH43 β-xylosidase from Geobacillus thermodenitrificans: Provides insights into the enzyme′s biophysical properties and its utility in biotechnological applications, furthering the understanding of D-(+)-Xylose′s role in enhancing enzyme performance under various industrial conditions (Melo et al., 2023).
- Genome-scale metabolic modeling reveals metabolic trade-offs associated with lipid production in Rhodotorula toruloides: Explores how D-(+)-Xylose can be used to optimize metabolic pathways for improved lipid production, highlighting its potential in synthetic biology and metabolic engineering to enhance bioproduct synthesis efficiency (Reķēna et al., 2023).
Other Notes
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service