PHR1998
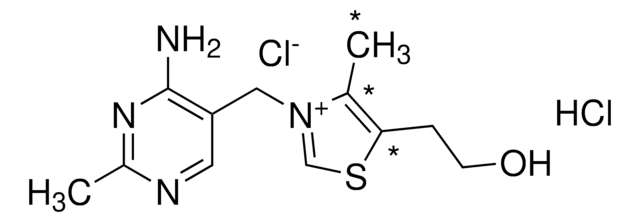
Thiamine Impurity C
Pharmaceutical Secondary Standard; Certified Reference Material
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
API family
thiamine
CofA
current certificate can be downloaded
packaging
pkg of 20 mg
format
neat
storage temp.
2-8°C
General description
Thiamine Impurity C is a pharmaceutical impurity of thiamine belonging to B group vitamins important for primary metabolism in all living organisms. Thiamine is widely used in the treatment or prevention of beriberi.
Application
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Thiamine may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using flow injection turbidimetric method and UV-Vis spectrophotometry.
Analysis Note
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Other Notes
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Footnote
To see an example of a Certificate of Analysis for this material enter LRAB8021 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Group Vitamins: Current Uses and Perspectives, 48(3), 659-667 (2018)
Flow injection turbidimetric determination of thiamine in pharmaceutical formulations using silicotungstic acid as precipitant reagent
Costa-Neto OC, et al.
Talanta, 48(3), 659-667 (1999)
Simultaneous determination and classification of riboflavin, thiamine, nicotinamide and pyridoxine in pharmaceutical formulations, by UV-visible spectrophotometry and multivariate analysis
Lopez-de-Alba LP, et al.
Journal of the Brazilian Chemical Society, 17(4), 715-722 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service