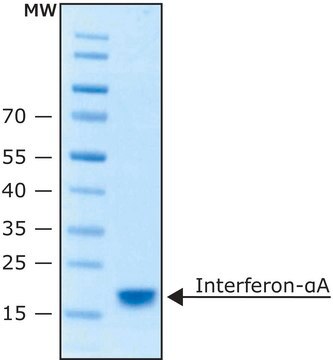
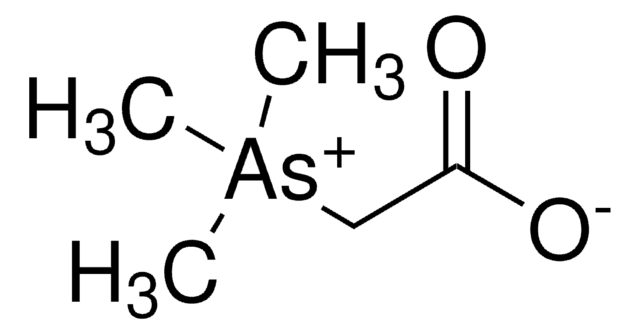
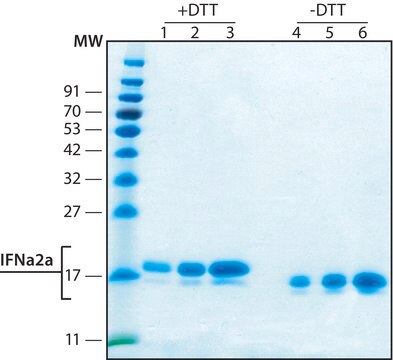
I0320301
Interferon α-2b
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
interferon
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Interferon α-2b EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
José María R Ingelmo et al.
European journal of obstetrics, gynecology, and reproductive biology, 167(2), 190-193 (2013-02-02)
A randomised and controlled experimental study was carried out to determine the effect of short and long series of treatment with recombinant human interferon-alpha-2b on surgically induced endometriosis in rats. Ninety-six Wistar adult female rats, which had undergone an autotransplant
L O Sakhno et al.
TSitologiia i genetika, 46(6), 12-18 (2013-01-05)
Spring rapeseed transgenic lines expressing human interferon alpha 2b were created by Agrobacterium-mediated transformation of aseptic plant leaf explants. The maximum antiviral activity of the leaf extracts reached 4500 IU/g fresh weight. It was determined that the antioxidant activity and
C Koh et al.
Alimentary pharmacology & therapeutics, 37(9), 887-894 (2013-03-07)
Although the short-term benefits of a sustained virological response (SVR) to interferon-based therapies of chronic hepatitis C (CHC) are well known, the long-term consequences of SVR are less clear. To assess changes in markers of disease activity and fibrosis in
Pierre L Triozzi et al.
Journal of translational medicine, 10, 241-241 (2012-12-12)
Blood biomarkers are needed to monitor anti-angiogenic treatments for cancer. The association of blood levels of microRNAs (miRs) implicated in angiogenesis with circulating endothelial cells (CEC) and with angiogenic proteins was examined in patients administered drugs with anti-angiogenic activity. Blood
Zhao-xia Ji et al.
Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology, 20(8), 617-620 (2012-12-05)
To investigate a baculovirus insect cell system for expressing an interferon alpha 2b (IFNa2b)/immunoglobulin G-4 (IgG4) Fc fusion protein, which has long-acting antiviral effects. Human IFNa2b and IgG4 Fc cDNAs were generated by molecular cloning and inserted into a baculovirus
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service