94194
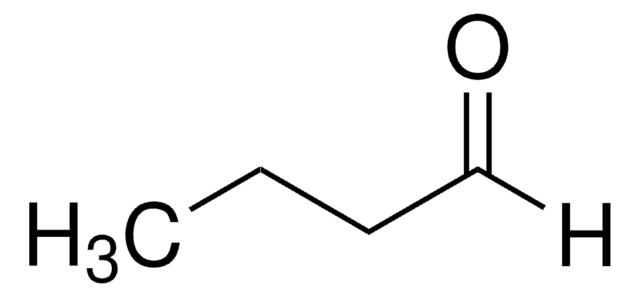
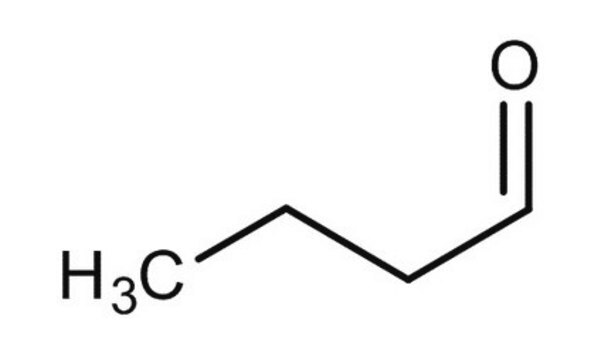
Butyraldehyde
analytical standard, contains ~0.1% 2,6-di-tert-butyl-4-methylphenol and ~1% water as stabilizer
Synonym(s):
Butanal
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor density
2.5 (vs air)
vapor pressure
90 mmHg ( 20 °C)
assay
≥97.0% (GC)
autoignition temp.
390 °F
shelf life
limited shelf life, expiry date on the label
contains
~0.1% 2,6-di-tert-butyl-4-methylphenol and ~1% water as stabilizer
expl. lim.
12.5 %
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.377-1.387
n20/D 1.380 (lit.)
bp
75 °C (lit.)
mp
−96 °C (lit.)
density
0.8 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
SMILES string
[H]C(=O)CCC
InChI
1S/C4H8O/c1-2-3-4-5/h4H,2-3H2,1H3
InChI key
ZTQSAGDEMFDKMZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Air samples by pentafluorophenyl hydrazine derivatization followed by analysis using gas chromatography (GC) equipped with flame ionization detector (FID).
- Ambient air by GC coupled to mass spectrometry (MS).
It may be used as an analytical reference standard for the analysis of BA in:
- Environmental samples by 2,4-dinitrophenylhydrazine derivatization followed by analysis using rapid resolution liquid chromatography-ultraviolet diode array detector (RRLC-UV-DAD) and RRLC coupled to ion-trap mass spectrometer (IT-MS) operating under the atmospheric pressure chemical ionization (APCI) mode.
- Saliva samples by magnetic-stirring-assisted micro-solid-phase extraction (μ-SPE) and headspace GC combined with ion-mobility spectrometry (IMS).
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
50.0 °F - Pensky-Martens closed cup
flash_point_c
< 10 °C - Pensky-Martens closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service