64643
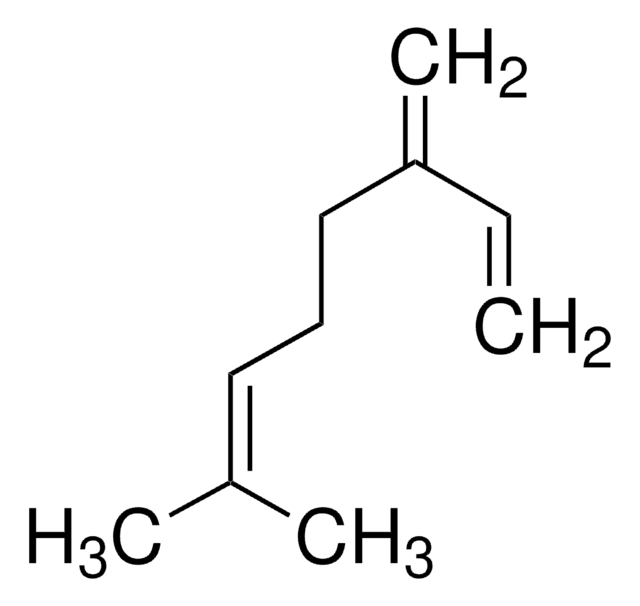
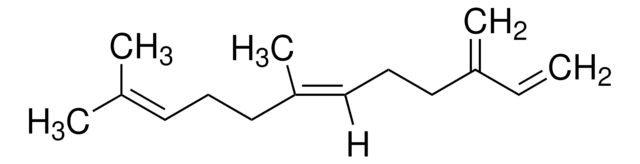
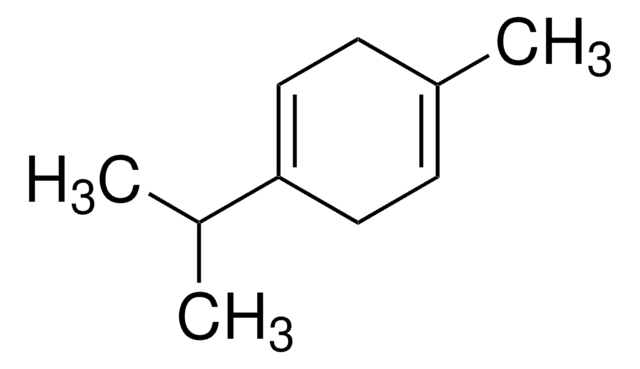
Myrcene
analytical standard
Synonym(s):
β-Myrcene, 7-Methyl-3-methylene-1,6-octadiene
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor density
4.7 (vs air)
vapor pressure
~7 mmHg ( 20 °C)
assay
≥90.0% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.469 (lit.)
bp
167 °C (lit.)
density
0.791 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
−20°C
SMILES string
C\C(C)=C/CCC(=C)C=C
InChI
1S/C10H16/c1-5-10(4)8-6-7-9(2)3/h5,7H,1,4,6,8H2,2-3H3
InChI key
UAHWPYUMFXYFJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Application
- Determination of four flavor compounds— isoamyl acetate, ethyl hexanoate, benzaldehyde, and myrcene, in commercial beer samples by using two solvent-less sample treatment techniques of stir-bar sorptive extraction (SBSE) and solid-phase microextraction (SPME) for their subsequent analysis by gas chromatography-flame ionization detection (GC-FID)
- Analysis of fresh mano samples for the detection and quantification of myrcene using a quartz crystal microbalance (QCM) sensor, modified with ethyl cellulose
- Multi-residue analysis of volatiles and fatty acids found in wild and cultivated fennel samples by a single extraction method and gas chromatographic-flame ionization detection (GC-FID)
- Identification and determination of volatile aroma compounds, commonly present in three plant species from the Citrus genus by using simultaneous distillation extraction (SDE) technique for sample treatment and analysis by gas chromatography-mass spectrometry (GC-MS) in electron ionization mode (EI)
- Secondary metabolite profiling of various plant parts collected from 82 plants belonging to 21 different cannabis strains using gas chromatography-mass spectrometry (GC-MS) for sterols and terpenoids (mono-, sesqui-, tri-), and high-performance liquid chromatography (HPLC) with UV and mass spectrometric (MS) detection for flavonoids
Packaging
Other Notes
signalword
Danger
hcodes
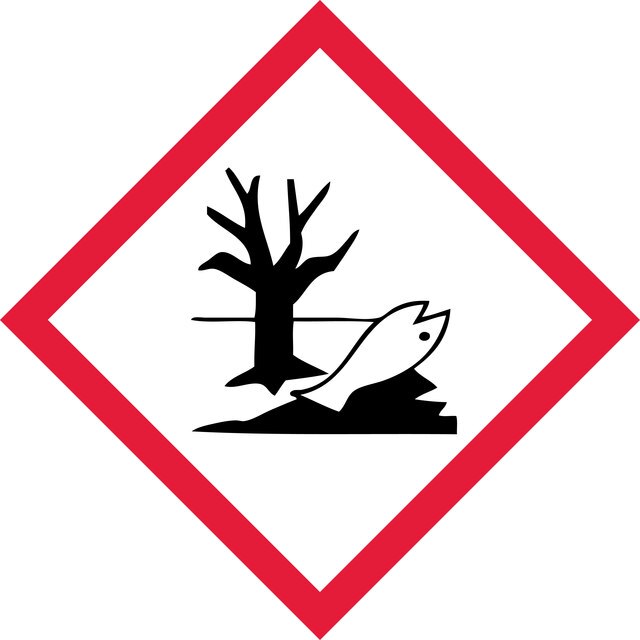
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
wgk_germany
WGK 3
flash_point_f
closed cup
flash_point_c
closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocols
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service