271012
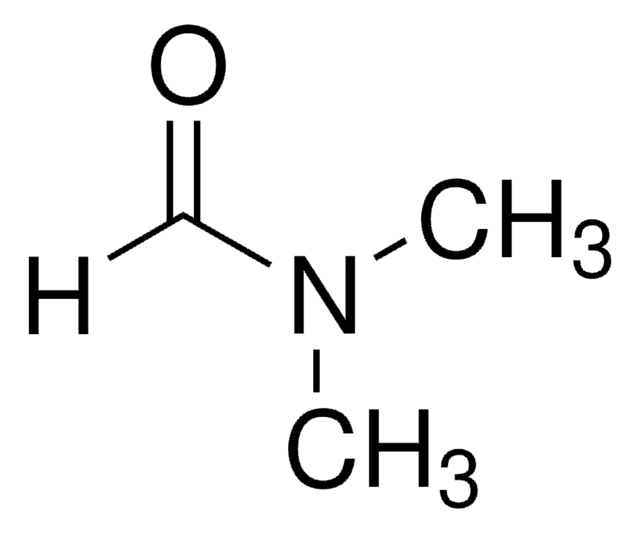
N,N-Dimethylacetamide
anhydrous, 99.8%
Synonym(s):
DMAc
About This Item
Recommended Products
grade
anhydrous
Quality Level
vapor density
3 (vs air)
vapor pressure
2 mmHg ( 25 °C)
4 mmHg ( 38 °C)
assay
99.8%
form
liquid
autoignition temp.
914 °F
expl. lim.
1.8 %, 100 °F
11.5 %, 160 °F
impurities
<0.005% water
evapn. residue
<0.001%
refractive index
n20/D 1.437 (lit.)
pH
4 (20 °C, 200 g/L)
bp
164.5-166 °C (lit.)
mp
−20 °C (lit.)
density
0.937 g/mL at 25 °C (lit.)
SMILES string
CN(C)C(C)=O
InChI
1S/C4H9NO/c1-4(6)5(2)3/h1-3H3
InChI key
FXHOOIRPVKKKFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Palladium (II) acetate-butyldi-1-adamantylphosphine catalyzed arylation of 2-isobutylthiazole with chlorobenzene to form 5-phenyl-2-isobutylthiazole.
- Synthesis of β-, γ-, and δ-metallo ester intermediates that can react with acid chlorides to form γ-, δ-, and ε-keto esters.
- Synthesis of (R)-(-)-methyl 1,1′-binaphthyl-2,2′-diylphosphate that can be used as a precursor for preparing (R)-(+)-1,1′-binaphthalene-2,2′-diol.
Packaging
Other Notes
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Repr. 1B
wgk_germany
WGK 2
flash_point_f
147.2 °F
flash_point_c
64 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service