17850
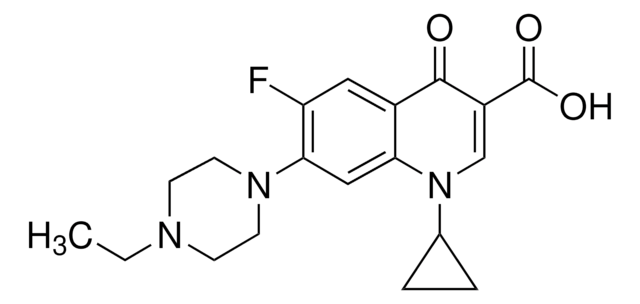
Ciprofloxacin
≥98% (HPLC)
Synonym(s):
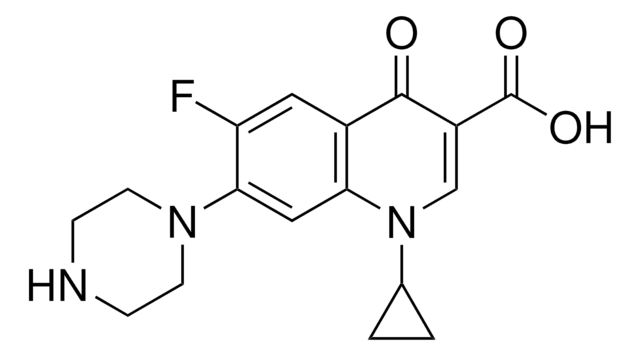
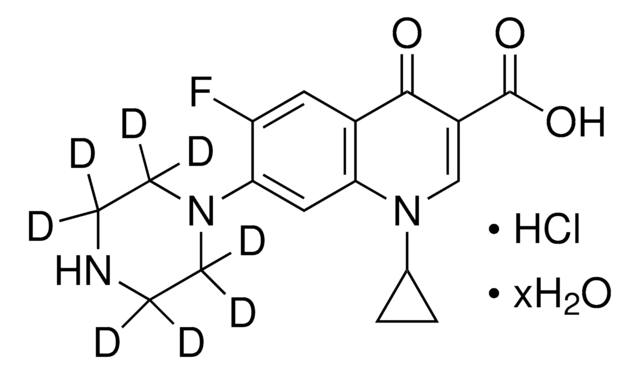
1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid, Ciprobay
About This Item
Recommended Products
agency
EPA 1694
Quality Level
assay
≥98% (HPLC)
form
powder or crystals
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
application(s)
environmental
mode of action
DNA synthesis | interferes
enzyme | inhibits
SMILES string
OC(=O)C1=CN(C2CC2)c3cc(N4CCNCC4)c(F)cc3C1=O
InChI
1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24)
InChI key
MYSWGUAQZAJSOK-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544) , KCNH1(3756)
rat ... Gabra1(29705)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chemical structure: fluoroquinolone
Application
Biochem/physiol Actions
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service