1.00247
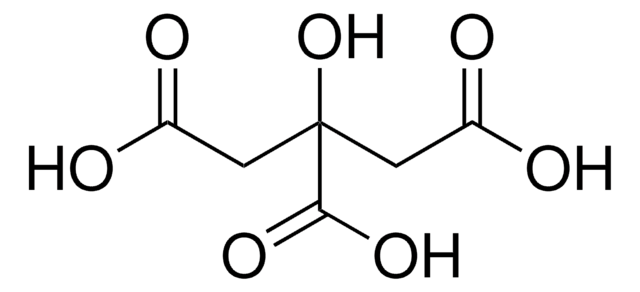
Citric acid
anhydrous fine-granular, EMPROVE® ESSENTIAL, Ph. Eur., BP, ChP, JP, USP, FCC, E 330
Pharma Manufacturing
Synonym(s):
2-Hydroxypropane-1,2,3-tricarboxylic acid, Hydroxytricarballylic acid
About This Item
Recommended Products
agency
BP
ChP
JP
Ph. Eur.
USP
Quality Level
reg. compliance
FCC
vapor pressure
<0.1 hPa ( 20 °C)
product line
EMPROVE® ESSENTIAL
assay
99.5-100.5% substance (anhydrous) basis (alkalimetric)
form
coarse powder
potency
3000 mg/kg LD50, oral (Rat)
expl. lim.
8 %, 65 °F
pH
1.7 (20 °C, 100 g/L in H2O)
pKa
(1) 3.13, (2) 4.76, (3) 6.4
bp
200 °C/1013 hPa (decomposition)
mp
153-159 °C (lit.)
solubility
water: 1330 g/L at 20 °C
density
1.665 g/cm3 at 18 °C
cation traces
Al: ≤0.2 ppm
As: ≤1 ppm
Hg: ≤1 ppm
Pb: ≤0.5 ppm
heavy metals (as Pb): ≤5 ppm
application(s)
liquid formulation
ophthalmics
pharmaceutical
semi-solid formulation
solid formulation
storage temp.
2-30°C
SMILES string
OC(=O)CC(O)(CC(O)=O)C(O)=O
InChI
1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
InChI key
KRKNYBCHXYNGOX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
Application
Citric acid anhydrous powder Emprove® Essential is citric acid present in its water-free, fine granulated form.
Legal Information
also commonly purchased with this product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service