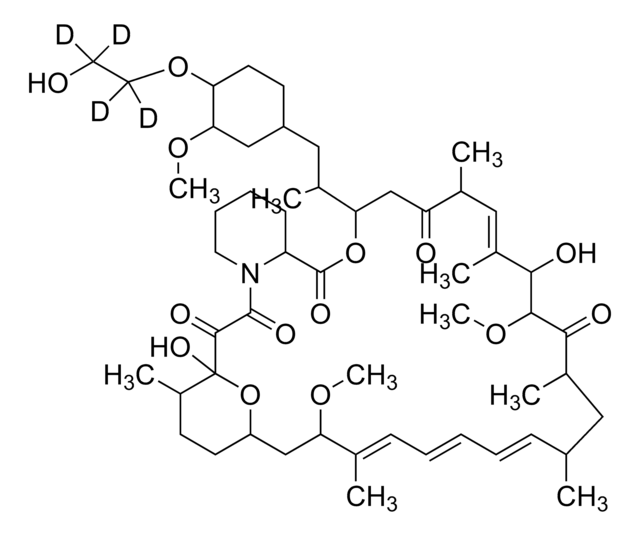
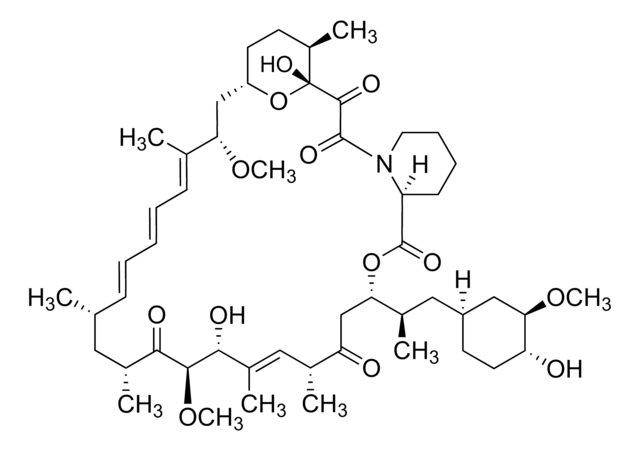
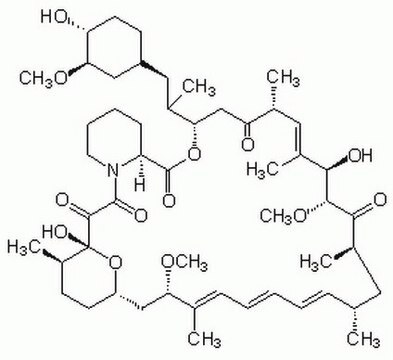
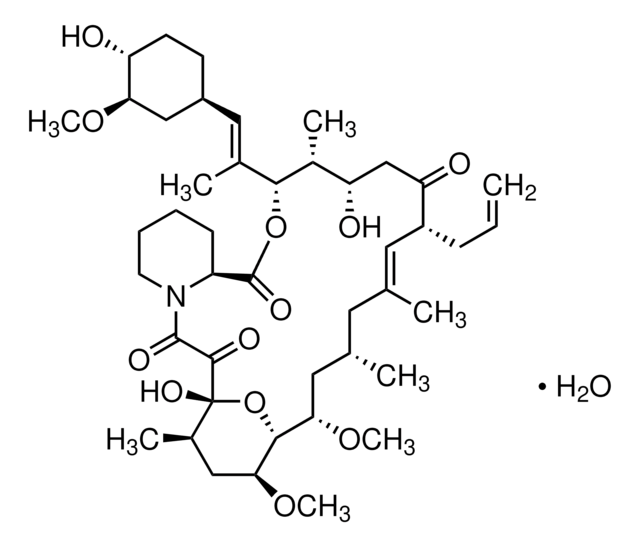
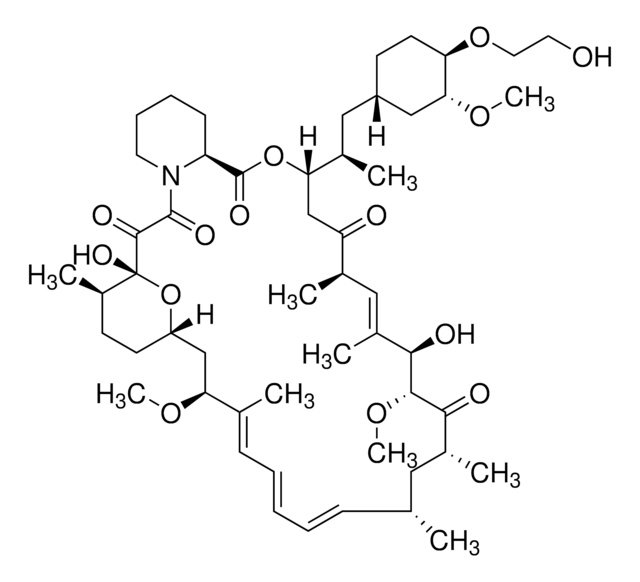
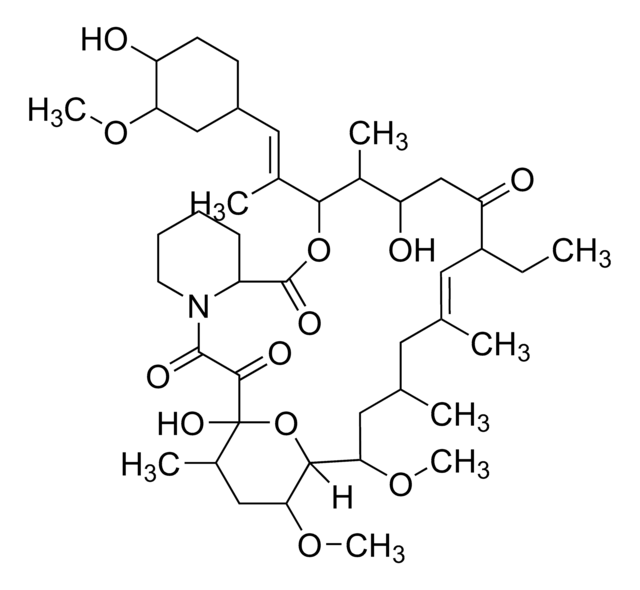
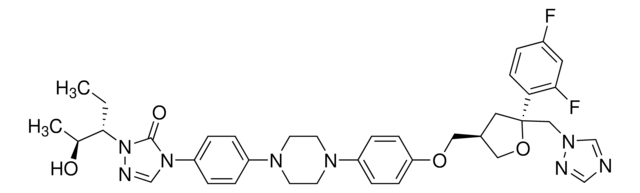
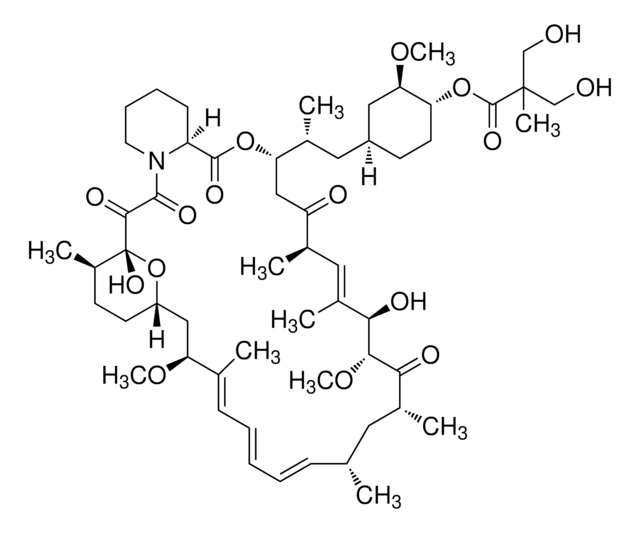
E-068
Everolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
(Snap-N-Spike®)
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
shipped in
dry ice
storage temp.
−70°C
InChI
1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
InChI key
HKVAMNSJSFKALM-GKUWKFKPSA-N
Gene Information
human ... FKBP1A(2280)
Related Categories
General description
Application
- Everolimus as a therapy for hepatoblastoma: Research demonstrates that Everolimus can induce autophagy-dependent ferroptosis in hepatoblastoma cells, highlighting its potential as a therapeutic agent in oncology research. This study provides insight into the mechanisms by which Everolimus can be utilized to target cancer cells through cell death pathways (Huang et al., 2024).
- Understanding oral mucosal injuries from mTOR inhibitors: A new hypothesis posits that oral mucosal injuries associated with mTOR inhibitors like Everolimus result from disruptions in cellular stress and apoptotic pathways. This study underscores the importance of understanding side effects in the context of targeted therapy for conditions such as cancers and immunosuppression (Sonis and Villa, 2023).
- Micellar formulation of Everolimus for neurological disorders: A stable micellar formulation of Everolimus (RAD001) has been developed for intracerebroventricular delivery, aimed at treating Alzheimer′s Disease and other neurological disorders. This formulation allows for direct brain administration, potentially enhancing the drug′s efficacy and safety profile (Gianessi et al., 2023).
- Pharmacokinetics in epilepsy treatment: The population pharmacokinetics of Everolimus were studied in patients with seizures associated with focal cortical dysplasia. This research aids in understanding the drug′s behavior in a specific neurological context, providing a foundation for dosing adjustments and therapeutic monitoring (Park et al., 2023).
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
wgk_germany
WGK 2
flash_point_f
35.6 °F
flash_point_c
2.0 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service