B-006
Butalbital solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
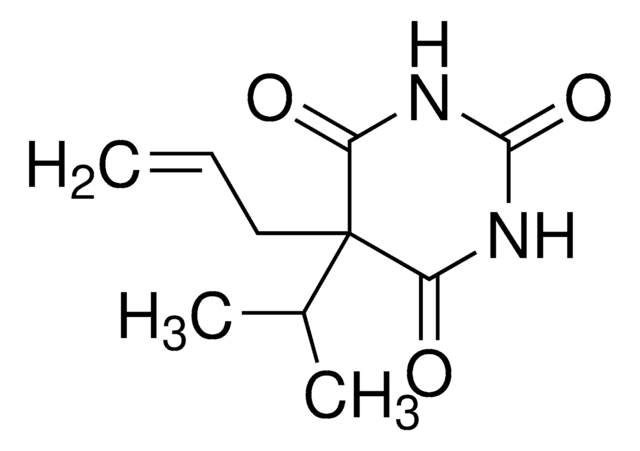
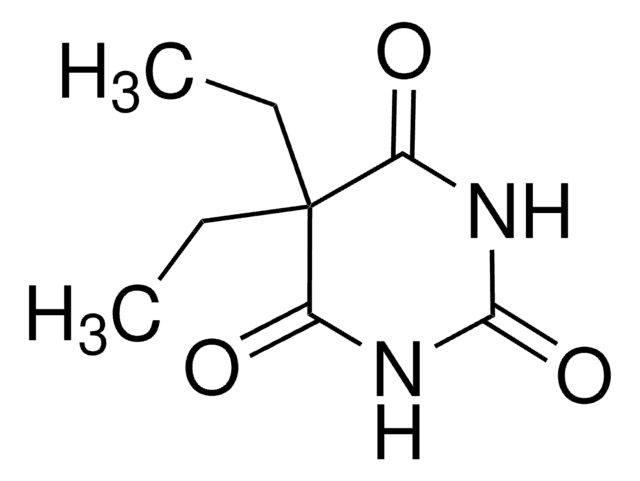
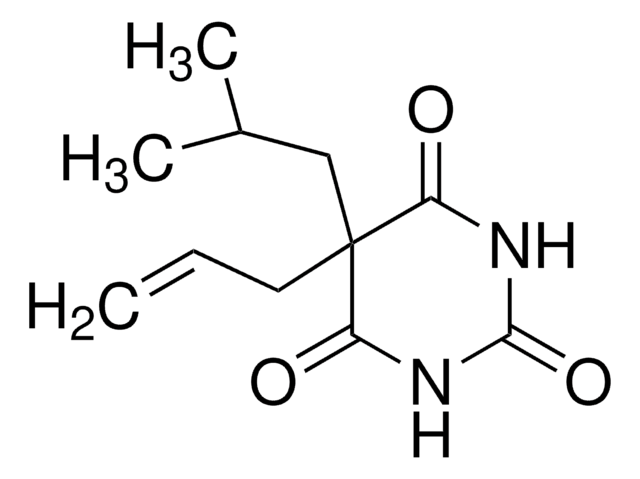
5-Allyl-5-isobutylbarbituric acid
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
SNAP-N-SPIKE®, SNAP-N-SHOOT®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIC (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
2-8°C
SMILES string
CC(C)CC1(CC=C)C(=O)NC(=O)NC1=O
InChI
1S/C11H16N2O3/c1-4-5-11(6-7(2)3)8(14)12-10(16)13-9(11)15/h4,7H,1,5-6H2,2-3H3,(H2,12,13,14,15,16)
InChI key
UZVHFVZFNXBMQJ-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Butalbital API for Research: Investigates Butalbital as an active pharmaceutical ingredient (API), focusing on its properties and applications in synthesizing complex pharmaceutical formulations, essential for developing new barbiturate-based treatments (Yang et al., 2022).
- Butalbital Compound Synthesis: Explores the synthesis of Butalbital and its derivatives, underlining its critical role in the development of CNS depressants and its pharmacological potential in neurobiological research (Marmura et al., 2015).
- Barbiturate Biochemical Research: Focuses on Butalbital′s effects on human platelet aggregation, offering insights into its mechanism of action within the cardiovascular system, which is vital for tailoring barbiturate-based therapies (Sato et al., 2003).
- Butalbital Pharmacological Studies: Discusses the pharmacological profiling of Butalbital, emphasizing its utility in managing acute pain and headaches, thereby supporting its continued use in clinical settings for migraine and tension headache relief (Mazer-Amirshahi et al., 2014).
- CNS Depressant Research Chemical: Analyzes Butalbital′s role as a central nervous system depressant, assessing its effectiveness and safety profile, which is crucial for its application in chronic pain management and treatment of cluster headaches (Cutrer et al., 1999).
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service