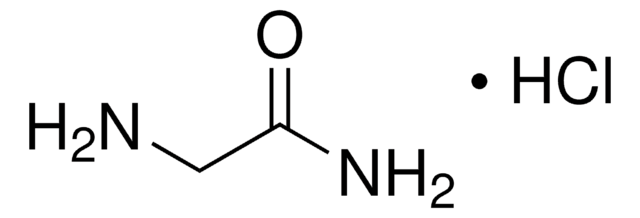
G6104
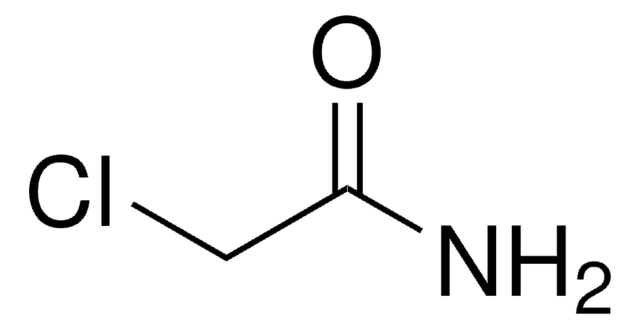
Glycinamide hydrochloride
98%
Synonym(s):
2-Aminoacetamide hydrochloride, Aminoacetamide hydrochloride, Glycine amide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2CH2CONH2 · HCl
CAS Number:
Molecular Weight:
110.54
Beilstein/REAXYS Number:
3554199
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
204 °C (dec.) (lit.)
SMILES string
Cl.NCC(N)=O
InChI
1S/C2H6N2O.ClH/c3-1-2(4)5;/h1,3H2,(H2,4,5);1H
InChI key
WKNMKGVLOWGGOU-UHFFFAOYSA-N
Related Categories
Application
Buffer useful in the physiological pH range.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gottfried K Schroeder et al.
Biochemistry, 46(13), 4037-4044 (2007-03-14)
As a model for mechanistic comparison with peptidyl transfer within the ribosome, the reaction of aqueous glycinamide with N-formylphenylalanine trifluoroethyl ester (fPhe-TFE) represents an improvement over earlier model reactions involving Tris. The acidity of trifluoroethanol (pKa 12.4) resembles that of
Eric Loeser et al.
Analytical chemistry, 79(14), 5382-5391 (2007-05-29)
When mobile-phase salt content is increased, cationic analytes often show increased retention. This effect is generally attributed to chaotropic or ion pairing effects. However, a cation exclusion mechanism could explain the same effects. In this study, experimental conditions were manipulated
Brett C Bookser et al.
Journal of medicinal chemistry, 48(24), 7808-7820 (2005-11-24)
4-(Phenylamino)-5-phenyl-7-(5-deoxy-beta-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine 1 and related compounds known as "diaryltubercidin" analogues are potent inhibitors of adenosine kinase (AK) and are orally active in animal models of pain such as the rat formalin paw model (GP3269 ED50= 6.4 mg/kg). However, the utility of
Xiaodong Jia et al.
The Journal of organic chemistry, 78(18), 9450-9456 (2013-08-21)
A catalytic α-sp(3) C-H oxidation of peptides and glycine amides was achieved under radical cation salt catalysis in the presence of O2, producing a series of substituted quinolines. The scope of this reaction shows good functional group tolerance and high
Hong Zhao et al.
Bioconjugate chemistry, 17(2), 341-351 (2006-03-16)
The utility of PEGylation for improving therapeutic protein pharmacology would be substantially expanded if the authentic protein drugs could be regenerated in vivo. Diminution of kinetic constants of both enzymes and protein ligands are commonly encountered following permanent bioconjugation with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service