C95501
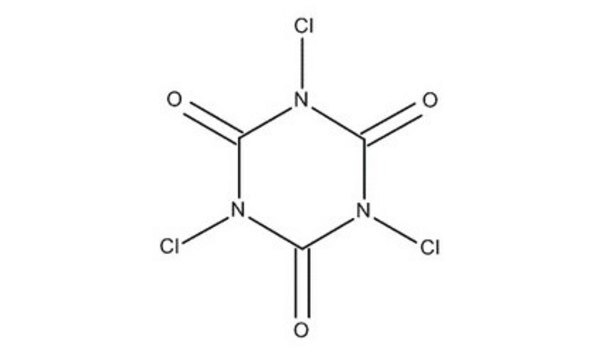
Cyanuric chloride
99%
Synonym(s):
2,4,6-Trichloro-1,3,5-triazine
About This Item
Recommended Products
vapor density
6.36 (vs air)
Quality Level
vapor pressure
0.8 mmHg ( 62.2 °C)
assay
99%
form
powder
bp
190 °C (lit.)
mp
145-147 °C (lit.)
storage temp.
2-8°C
SMILES string
Clc1nc(Cl)nc(Cl)n1
InChI
1S/C3Cl3N3/c4-1-7-2(5)9-3(6)8-1
InChI key
MGNCLNQXLYJVJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- In the preparation of acyl azides from carboxylic acids and sodium azide.
- For the conversion of carboxylic acids, N-Boc, N-Cbz, and N-Fmoc amino acids into corresponding alcohols.
- For the conversion of alcohols into the corresponding carbonyl compounds by alternative Swern oxidation reaction.
It can also be employed as a catalyst in the Beckmann rearrangement of ketoximes into amides in the presence of ZnCl2.
signalword
Danger
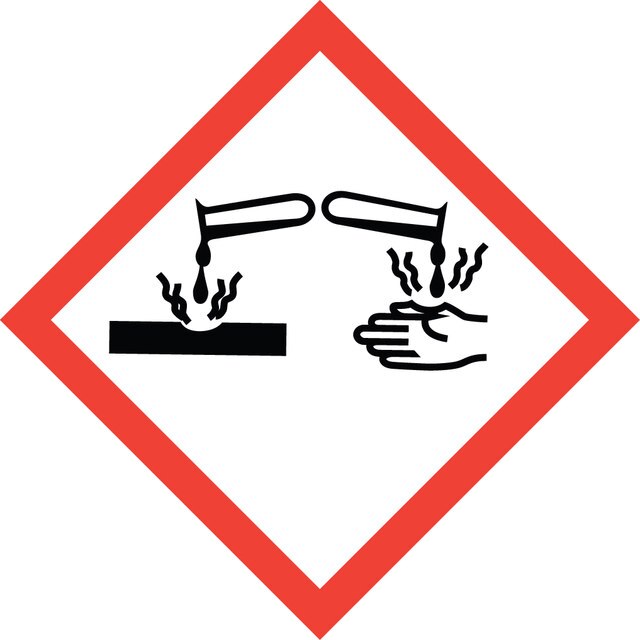
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A - STOT SE 3
target_organs
Respiratory system
supp_hazards
Storage Class
6.1B - Non-combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 1
flash_point_f
392.0 °F - closed cup
flash_point_c
> 200 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Collagen molecules play a critical role in tissue architecture and strength, and in cell-matrix interactions as insoluble ligands to regulate the diverse phenotypic activities of cells.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service