C4706
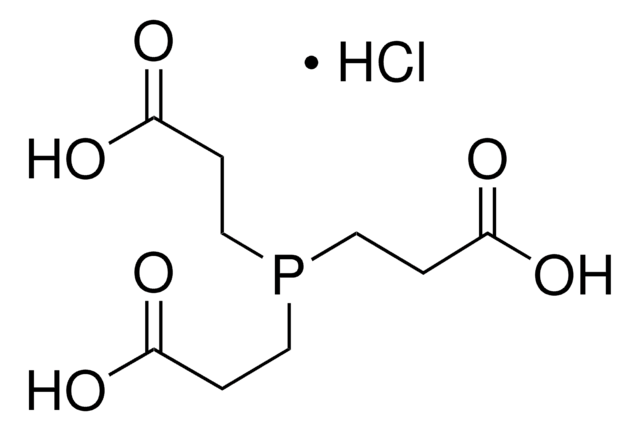
Tris(2-carboxyethyl)phosphine hydrochloride
powder
Synonym(s):
TCEP
About This Item
Recommended Products
description
Protect from moisture
Quality Level
form
powder
reaction suitability
reagent type: reductant
color
white
solubility
H2O: 50 mg/mL
storage temp.
2-8°C
SMILES string
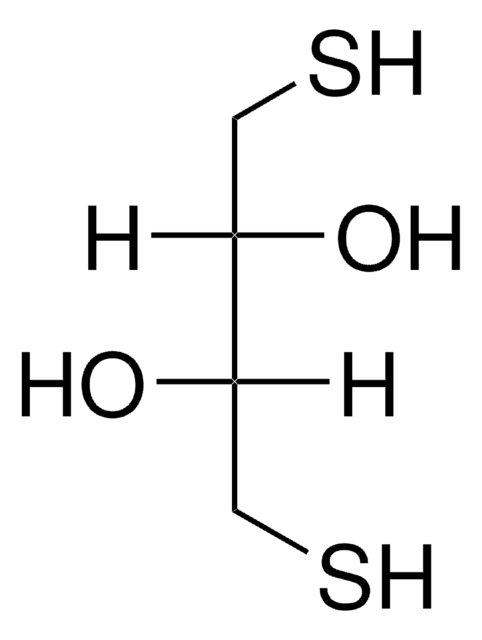
Cl[H].OC(=O)CCP(CCC(O)=O)CCC(O)=O
InChI
1S/C9H15O6P.ClH/c10-7(11)1-4-16(5-2-8(12)13)6-3-9(14)15;/h1-6H2,(H,10,11)(H,12,13)(H,14,15);1H
InChI key
PBVAJRFEEOIAGW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a reducing agent for the reduction of sulfoxides, sulfonyl chlorides, N-oxides, and azides. It can also be used in azide-alkyne cycloaddition reaction in the presence of a copper catalyst.
- To reduce disulfide bonds in various proteins.
- As a reagent for the selective reduction of disulfides in water.
- To remove ruthenium-derived metathesis catalysts via aqueous washing of a crude reaction mixture when it is basified.
- As a reducing agent for the reduction of various alkyl disulfides such as trans-4,5-dihydroxy-1,2-dithiane.
Caution
related product
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
wgk_germany
WGK 1
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service