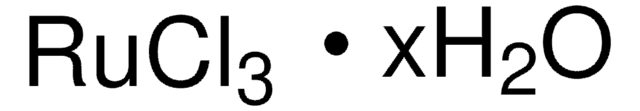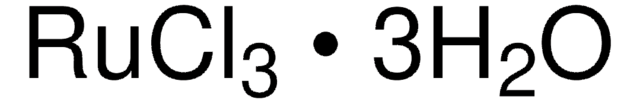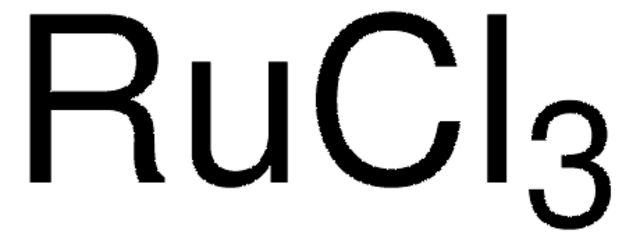931578
Ruthenium(III) chloride hydrate
≥99.9% trace metals basis
Synonym(s):
Ruthenium trichloride, Trichlororuthenium hydrate
About This Item
Recommended Products
Quality Level
assay
≥99.9% trace metals basis
form
powder
impurities
≤1000.0 ppm (trace metals analysis)
solubility
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
density
3.11 g/cm3
SMILES string
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
InChI key
BIXNGBXQRRXPLM-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
Application
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible, corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service