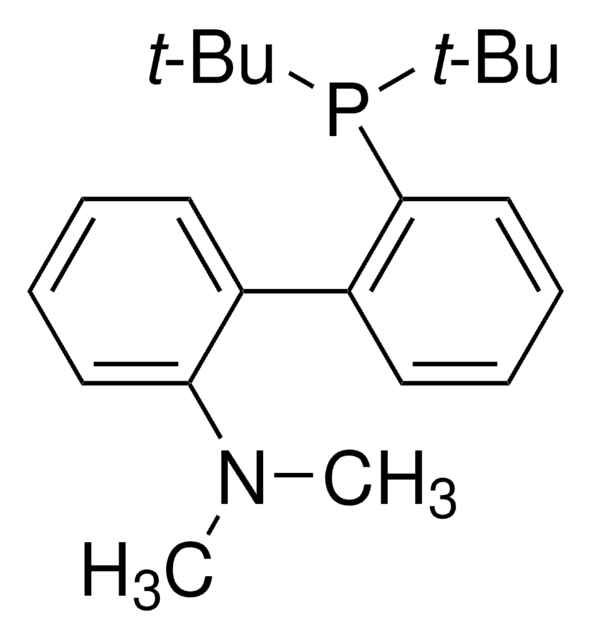
638439
JohnPhos
97%
Synonym(s):
(2-Biphenyl)di-tert-butylphosphine, (2-Biphenylyl)di-tert-butylphosphine, 2-(Di-tert-butylphosphino)biphenyl
About This Item
Recommended Products
Quality Level
assay
97%
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
86-88 °C (lit.)
functional group
phosphine
SMILES string
CC(C)(C)P(c1ccccc1-c2ccccc2)C(C)(C)C
InChI
1S/C20H27P/c1-19(2,3)21(20(4,5)6)18-15-11-10-14-17(18)16-12-8-7-9-13-16/h7-15H,1-6H3
InChI key
CNXMDTWQWLGCPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Learn more about Buchwald Phosphine Ligands
Application
- Hydrophenoxylation of unactivated internal alkynes.
- Microwave-mediated Suzuki-Miyaura cross-coupling of benzylic bromides.
- Pharmaceutical synthesis of novel imidazo[1,2-a]pyridines, having potent activity against the herpes virus.
- Barluenga′s coupling of vinyl bromides with hydrazines.
- Pd-catalyzed 2,3-diarylation of α,α-disubstituted-3-thiophenemethanols, via cleavage of C-H and C-C bonds.
Catalyst for:
- Decarboxylative cross-coupling of dialkoxybenzoic acids with diaryl disulfides or diaryl diselenides
- Stereoselective preparation of imidazolidinones via intramolecular hydroamination of N-allylic-N-arylureas
- Regioselective arylation of olefins with aryl chlorides
- Cross-coupling reaction for the synthesis of polyunsaturated macrolactones
- Regioselective O-alkylation reactions
- Sonogashira-type cross coupling
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald Ligands
The Pd-catalyzed C–N bond formation has become an important synthetic reaction in the past 20 years. Several research groups have investigated this reaction and developed very versatile catalysts. Buchwald and coworkers have been very active in synthesizing and developing a portfolio of phosphine ligands for this transformation and other cross-coupling reactions.
Buchwald Phosphine Ligands
Over the past several years, Pd-catalyzed cross-coupling of silicon compounds has rapidly gained acceptance as a suitable alternative to more commonly known methods such as: Stille (Sn), Kumada (Mg), Suzuki (B), and Negishi (Zn) cross-couplings.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)