567159
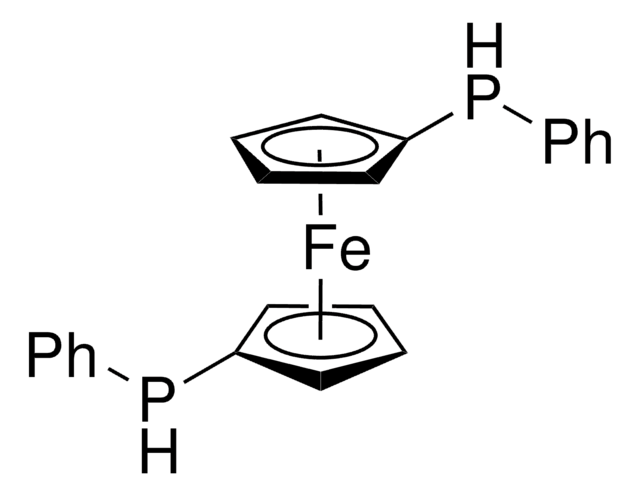
1,1′-Bis(phenylphosphinidene)ferrocene
97%
Synonym(s):
1,1′-(Ferrocenediyl)phenylphosphine
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
mp
88-92 °C (lit.)
functional group
phosphine
storage temp.
2-8°C
SMILES string
[Fe].[CH]1[CH][CH][C]([CH]1)P([C]2[CH][CH][CH][CH]2)c3ccccc3
InChI
1S/C16H13P.Fe/c1-2-8-14(9-3-1)17(15-10-4-5-11-15)16-12-6-7-13-16;/h1-13H;
InChI key
HKOUDNXXOXEYNZ-UHFFFAOYSA-N
Related Categories
Application
- Preparation of macrocyclic tridentate ferrocenylphosphine ligands
- Preparation of phosphorus-bridged ferrocenophane with syn and anti conformations
- Reaction with phenyllithium and chlorophosphine to give ferrocenediyl ligand and its rhodium complex catalyst for selective hydroformylation of alkenes
- Ring-opening reactions via ring slippage
- Preparation of palladium chloro (ferrocene-bridged)phosphine-phosphonite complex for catalysis of Heck reaction of iodobenzene with Me acrylate
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(S)[(Sp)-2-(Diphenylphosphino)ferrocenyl]-4-isopropyloxazoline 97%](/deepweb/assets/sigmaaldrich/product/structures/265/471/6ec7300c-126b-4e22-9b5b-12634da58dbd/640/6ec7300c-126b-4e22-9b5b-12634da58dbd.png)