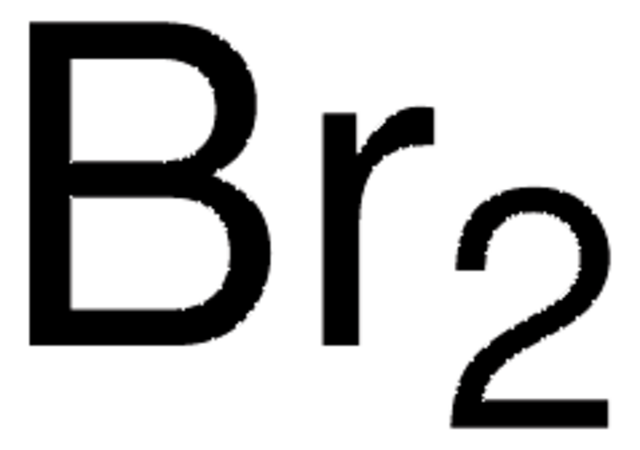470864
Bromine
≥99.99% trace metals basis
Synonym(s):
Dibromine
About This Item
Recommended Products
vapor density
7.14 (vs air)
Quality Level
vapor pressure
175 mmHg ( 20 °C)
671 mmHg ( 55 °C)
assay
≥99.5% (by Na2S2O3, titration)
≥99.99% trace metals basis
resistivity
7.8E18 μΩ-cm, 20°C
impurities
≤0.001% I2
≤0.001% S compounds
<100 ppm total metallic impurities
evapn. residue
≤0.005%
bp
58.8 °C (lit.)
mp
−7.2 °C (lit.)
density
3.119 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤0.05%
cation traces
Ni: ≤5 ppm
heavy metals: ≤2 ppm
SMILES string
BrBr
InChI
1S/Br2/c1-2
InChI key
GDTBXPJZTBHREO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
It can be used:
- To brominate alkylbenzenes at the benzylic position using photocatalytic conditions.
- To prepare brominated aromatic compounds by electrophilic aromatic substitution reactions in the presence of Lewis acid catalysts.
- For the bromination of carbonyl compounds at a carbonyl group via Hell-Volhard-Zelinski reaction in the presence of phosphorus trihalides.
- To prepare isocyanates, carbamates, or amines by reacting with primary amides in the presence of base via Hofmann rearrangement.
Other Notes
signalword
Danger
hcodes
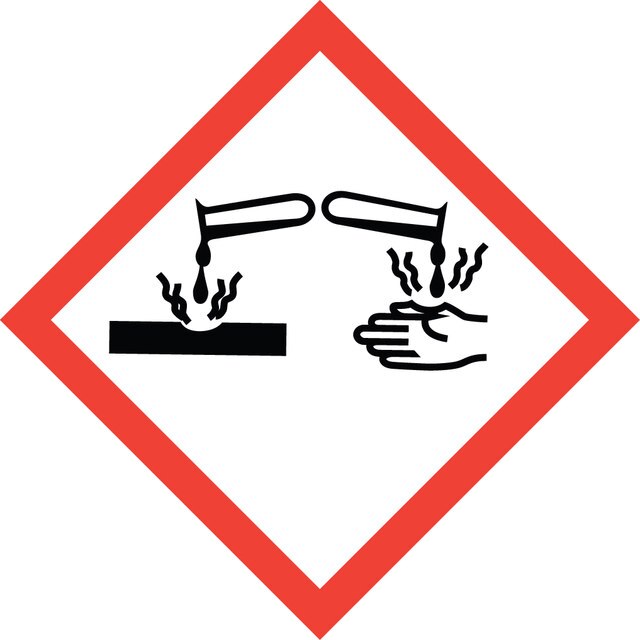
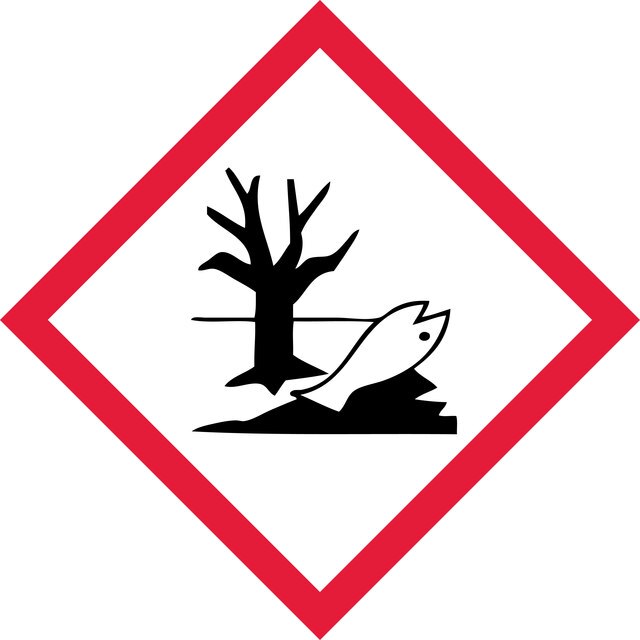
Hazard Classifications
Acute Tox. 1 Inhalation - Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1A
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service