291811
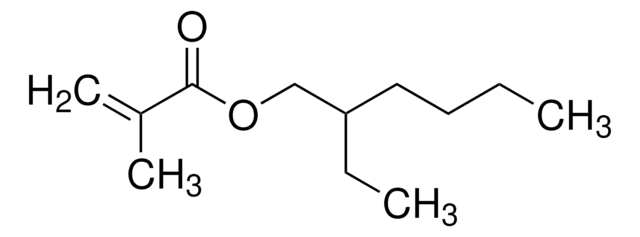
Lauryl methacrylate
contains 500 ppm MEHQ as inhibitor, 96%
Synonym(s):
Dodecyl 2-methyl-2-propenoate, Dodecyl methacrylate
About This Item
Recommended Products
Quality Level
assay
96%
contains
500 ppm MEHQ as inhibitor
refractive index
n20/D 1.445 (lit.)
bp
142 °C/4 mmHg (lit.)
mp
−7 °C (lit.)
density
0.868 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCOC(=O)C(C)=C
InChI
1S/C16H30O2/c1-4-5-6-7-8-9-10-11-12-13-14-18-16(17)15(2)3/h2,4-14H2,1,3H3
InChI key
GMSCBRSQMRDRCD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- To prepare polymeric co-stabilizer by free-radical copolymerization.
- To fabricate fluorine-less superhydrophobic cotton fabrics via graft polymerization process.
- In the synthesis of prepolymers that are used in oil absorbents.
signalword
Warning
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
224.6 °F - closed cup
flash_point_c
107 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service