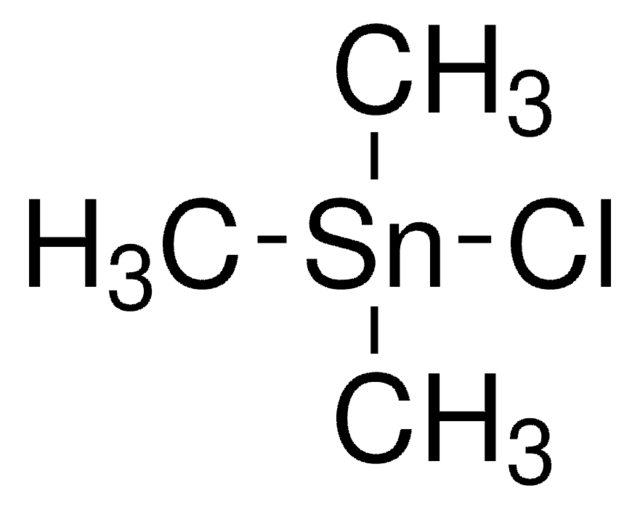146498
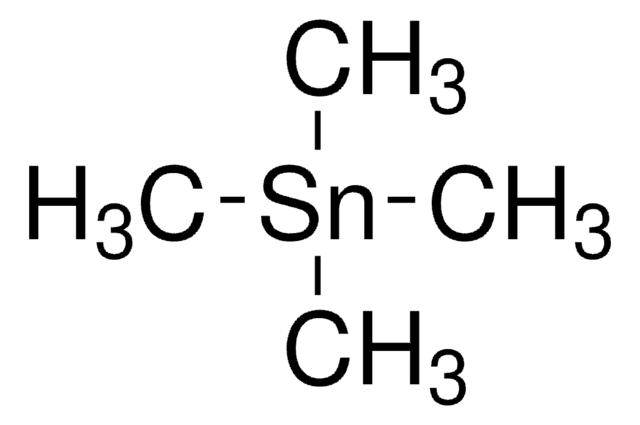
Trimethyltin chloride
Synonym(s):
Chlorotrimethylstannane
About This Item
Recommended Products
form
crystals
Quality Level
mp
37-39 °C (lit.)
SMILES string
C[Sn](C)(C)Cl
InChI
1S/3CH3.ClH.Sn/h3*1H3;1H;/q;;;;+1/p-1
InChI key
KWTSZCJMWHGPOS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
It can also be used as a reagent to prepare:
- Organotrimethyltin derivatives by reacting with organocopper compounds via transmetalation reaction.
- Acetophenone by palladium-catalyzed coupling reaction with benzoyl chloride.
- Optically active propargyl trimethylstannane by treating with chiral allenyltitanium.
- Trimethylstannyl nucleophiles, which are applicable in the formation of Sn-C bonds via SN2 reactions, SRN1 reactions, and halogen-metal exchanges.
- Carbocycles by reacting with unactivated dienes or trienes via radical-mediated carbocyclization reaction in the presence of NaBH3CN and a catalytic amount of AIBN.
Me3SnCl can also be used as a Lewis acid catalyst in asymmetric allylic alkylation reactions.
signalword
Danger
Hazard Classifications
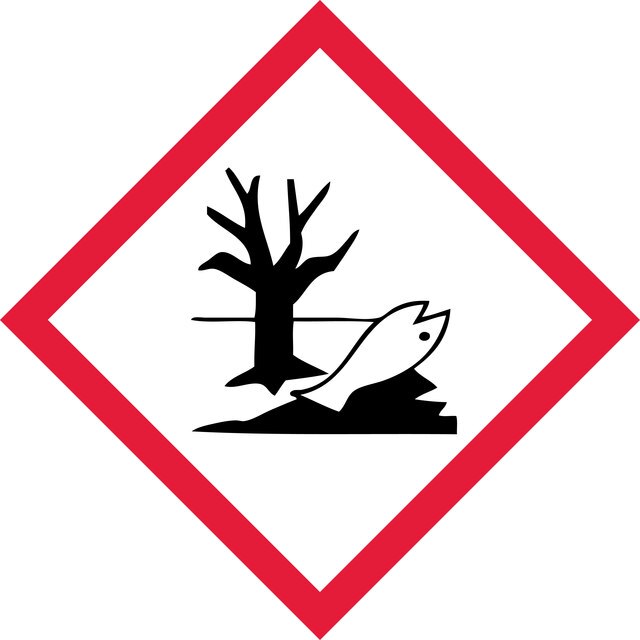
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
206.6 °F - closed cup
flash_point_c
97 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service