L4277
Lipase from Aspergillus oryzae
≥20,000 U/g
Synonym(s):
Palatase® 20,000L
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
lyophilized
Quality Level
specific activity
≥20,000 U/g
storage temp.
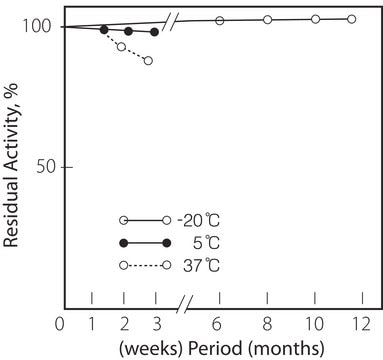
2-8°C
InChI
1S/C11H9N3O2.Na/c15-8-4-5-9(10(16)7-8)13-14-11-3-1-2-6-12-11;/h1-7,16H,(H,12,14);/q;+1/b13-9-;
InChI key
QWZUIMCIEOCSJF-CHHCPSLASA-N
General description
Rhizomucor miehei lipase is a carboxylesterase and belongs to the α/β-hydrolase family. It comprises a lid domain, hinge domain and a catalytic triad Ser?His?Asp/Glu. The three-dimensional structure is a α/β hydrolase fold which is very similar as that of esterase enzyme.
Application
Lipase from Rhizomuco miehei has been used:
- in the partial digestion of triacylglycerols (TAG)
- in the digestion of (12-ricinoleoylricinoleoyl)diricinoleoylglycerol (RRRR) present in castor oil
- in the hydrolysis of linseed oil
Biochem/physiol Actions
Lipase catalyzes the hydrolysis of monoacylglycerols and diacylglycerols and its activity is inhibited by divalent actions. Rhizomuco miehei lipase is an industrial enzyme with application in food, detergent and pharmaceutical industries. It is also used in immobilization studies for 1,3-dioleoyl-2-palmitoylglycerol (OPO) synthesis. Rhizomuco miehei lipase may have potential in producing biodiesel from waste cooking oil.
Preparation Note
purified 1,3-specific lipase from Rhizomucor miehei produced by submerged fermentation of a genetically modified Rhizomucor miehei microorganism
Legal Information
A product of Novozyme corp.
Palatase is a registered trademark of Novozymes Corp.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jiann-Tsyh Lin et al.
Journal of agricultural and food chemistry, 56(10), 3616-3622 (2008-04-30)
(12-Ricinoleoylricinoleoyl)diricinoleoylglycerol (RRRR), a tetraacylglycerol, was identified earlier in castor oil. Using ESI-MS (4), 95% of the 12-ricinoleoylricinoleoyl chain was identified at the sn-2 position of the glycerol backbone of RRRR. Regiospecific location of the 12-ricinoleoylricinoleoyl chain of RRRR on the
Xianming Zhao et al.
Frontiers in microbiology, 10, 645-645 (2019-04-12)
Thraustochytrium is a marine protist that can accumulate a large amount of very long chain polyunsaturated fatty acids (VLCPUFA) in triacylglycerols (TAG). How these freshly synthesized VLCPUFAs are channeled into TAG remains unknown. In this study, the glycerolipid profile of
Thais de Andrade Silva et al.
Scientific reports, 12(1), 6815-6815 (2022-04-28)
The use of enzymes immobilized on nanomagnetic supports has produced surprising results in catalysis, mainly due to the increase in surface area and the potential for recovery and reuse. However, the meticulous control of the process and difficulties in reproducibility
Preliminary Investigation: Stearidonic Acid Production by Genetically Modified Saccharomyces cerreviseae Using Linseed Oil as A Fatty Acid Source
Muzakhar K
Jurnal Ilmu Dasar, 9(1), 8-Jan-8-Jan (2008)
Andre Mong Jie Ng et al.
International journal of molecular sciences, 22(19) (2021-10-14)
Medium-chain triglycerides (MCTs) are an emerging choice to treat neurodegenerative disorders such as Alzheimer's disease. They are triesters of glycerol and three medium-chain fatty acids, such as capric (C8) and caprylic (C10) acids. The availability of C8-C10 methyl esters (C8-C10
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service