K4519
Keratinase
lyophilized powder
Synonym(s):
Keratinase from Bacillus licheniformis, KerA, Keratinolytic protease
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
General description
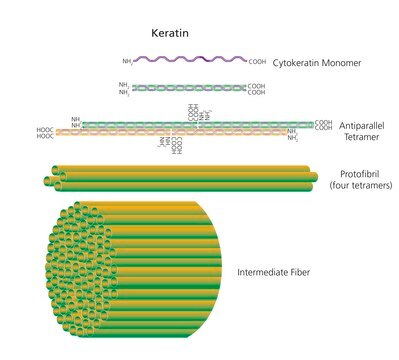
Keratinase is a non-specific serine protease that cleaves non-terminal peptide bonds. This enzyme is active between 37°- 70°C. The highest activity is typically seen at 70°C.
Keratinase is also metalloprotease present in bacteria as well as eukaryotes. Keratinase isolated from Bacilus licheniformis culture is a monomeric enzyme.
Application
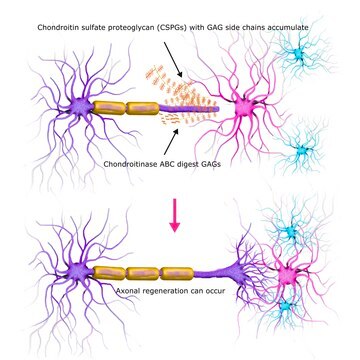
Keratinase may be used for enzymatic treatment of elementary body (EB), GAG molecules, and cells in the study of the role glycosaminoglycans (GAGs) in the invasion of host cells by Chlamydia pneumoniae strains.
Biochem/physiol Actions
Keratinase is a particular class of extracellular proteolytic inducible enzyme with the capability of degrading insoluble keratin substrates. It is important for hydrolyzing hair, feather, and collagen in sewage system during waste water treatment. It is also useful in food industry, animal feed preparation etc. Insoluble feather keratin from poultry industry may be converted by enzymatic hydrolysis to glues, feedstuffs, fertilizers, films or used for the production of rare amino acids serine, cysteine and proline.
Physical properties
Keratinase is activated by 0.10% SDS, 1.0% CTAB, and EDTA. Keratinase is partially inhibited by Tween®20, DMSO, isopropanol, methanol, and ethanol.
Unit Definition
One unit of enzyme is able to hydrolyze casein resulting in an absorbance value as the Folin-Ciocalteau reagent equivalent to 1 umole (181μg) of tyrosine per minute at pH 7.5 at 37 °C.
Preparation Note
The enzyme can be solubilized at 0.5-1.0 mg/mL in either sterile water or phosphate buffer. The best activity is seen with freshly prepared solutions. However, single-use aliquots of Keratinase solutions can be stored at -20° C.
Substrates
Keratin. Keratinases have also been used for the degradation of prion and prion-like proteins.
Other Notes
This enzyme contains a C-terminus 6-Histidine tag.
Legal Information
TWEEN is a registered trademark of Croda International PLC
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
X Lin et al.
Applied and environmental microbiology, 58(10), 3271-3275 (1992-10-01)
A keratinase was isolated from the culture medium of feather-degrading Bacillus licheniformis PWD-1 by use of an assay of the hydrolysis of azokeratin. Membrane ultrafiltration and carboxymethyl cellulose ion-exchange and Sephadex G-75 gel chromatographies were used to purify the enzyme.
Keratinolytic activity from the broth of a feather-degrading thermophillic Streptomyces thermoviolaceus strain SD8.
Chitte RR, Nalawade VK, Dey S.
Letters in Applied Microbiology, 28, 131-136 (1999)
Cheng-gang Cai et al.
Journal of Zhejiang University. Science. B, 9(1), 60-67 (2008-01-16)
A new feather-degrading bacterium was isolated from a local feather waste site and identified as Bacillus subtilis based on morphological, physiochemical, and phylogenetic characteristics. Screening for mutants with elevated keratinolytic activity using N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis resulted in a mutant strain KD-N2
Ellen J Beswick et al.
The Journal of infectious diseases, 187(8), 1291-1300 (2003-04-16)
The role of glycosaminoglycans (GAGs) in the invasion of host cells by Chlamydia pneumoniae strains TW-183 and A-03 was investigated and compared with the role of invasion by C. trachomatis serovars L2 and E. The quantities of epithelial and endothelial
Bacterial Keratinases: Useful Enzymes for Bioprocessing Agroindustrial Wastes and Beyond.
Brandelli A
Food and Bioprocess Technology, 1(2), 105-116 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service