H3398
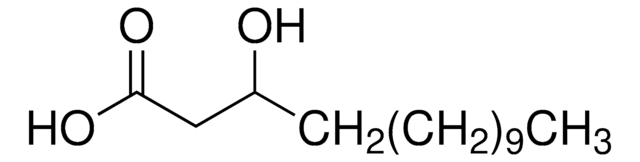
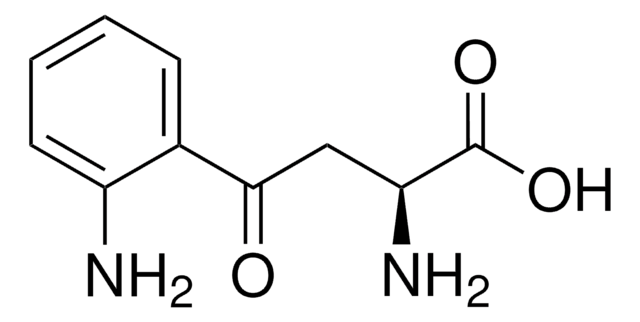
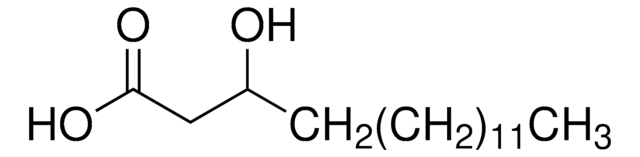
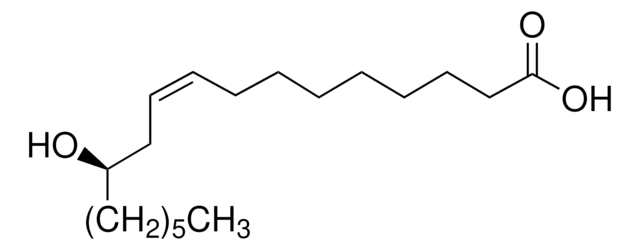
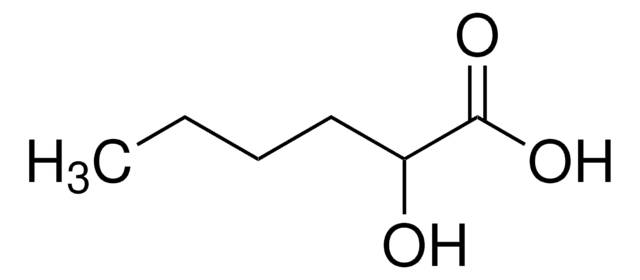
DL-β-Hydroxylauric acid
≥99% (GC)
Synonym(s):
3-Hydroxydodecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H24O3
CAS Number:
Molecular Weight:
216.32
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
Recommended Products
Quality Level
Assay
≥99% (GC)
form
solid
functional group
carboxylic acid
lipid type
saturated FAs
shipped in
ambient
storage temp.
2-8°C
SMILES string
CCCCCCCCCC(O)CC(O)=O
InChI
1S/C12H24O3/c1-2-3-4-5-6-7-8-9-11(13)10-12(14)15/h11,13H,2-10H2,1H3,(H,14,15)
InChI key
MUCMKTPAZLSKTL-UHFFFAOYSA-N
Biochem/physiol Actions
DL-3-Hydroxydodecanoic acid (3HDD) is a racemic mixture of D- and L-3HDD. 3-hydroxydodecanoic acid is found as a monomer in the construction of polyhydroxyalkanoates. 3HDD is used to study the roles of β-hydroxy fatty acids in chronic inflammation and insulin resistance and the biosynthesis of diffusible signal factors involved in quorum sensing.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I MacArthur et al.
Journal of bacteriology, 193(18), 4726-4735 (2011-07-19)
PagL and LpxO are enzymes that modify lipid A. PagL is a 3-O deacylase that removes the primary acyl chain from the 3 position, and LpxO is an oxygenase that 2-hydroxylates specific acyl chains in the lipid A. pagL and
Hongkai Bi et al.
Molecular microbiology, 83(4), 840-855 (2012-01-10)
Signal molecules of the diffusible signal factor (DSF) family have been shown recently to be involved in regulation of pathogenesis and biofilm formation in diverse Gram-negative bacteria. DSF signals are reported to be active not only on their cognate bacteria
Lina Yue et al.
Se pu = Chinese journal of chromatography, 24(1), 10-13 (2006-07-11)
To analyse the impurity of bacterium source of standard endotoxin, 3-hydroxy fatty acid species in different endotoxin standards was determined by gas chromatography/mass spectrometry (GC/MS) using N, O-bis (trimethylsilyl) trifluoroacetamide as the silanizing reagent. GC/MS analysis was performed using a
Structural basis for the sugar nucleotide and acyl-chain selectivity of Leptospira interrogans LpxA.
Lori I Robins et al.
Biochemistry, 48(26), 6191-6201 (2009-05-22)
The first step of lipid A biosynthesis is catalyzed by LpxA in Escherichia coli (EcLpxA), an acyltransferase selective for UDP-GlcNAc and R-3-hydroxymyristoyl-acyl carrier protein (ACP). Leptospira interrogans LpxA (LiLpxA) is extremely selective for R-3-hydroxylauroyl-ACP and an analogue of UDP-GlcNAc, designated
Ahleum Chung et al.
Applied microbiology and biotechnology, 83(3), 513-519 (2009-03-10)
To produce extracellular chiral 3-hydroxyacyl acids (3HA) by fermentation, a novel pathway was constructed by expressing tesB gene encoding thioesterase II into Pseudomonas putida KTOY01, which was a polyhydroxyalkanoate (PHA) synthesis operon knockout mutant. 3HA mixtures of 0.35 g/l consisting
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service