C7698
Cycloheximide
from microbial, ≥94% (TLC)
Synonym(s):
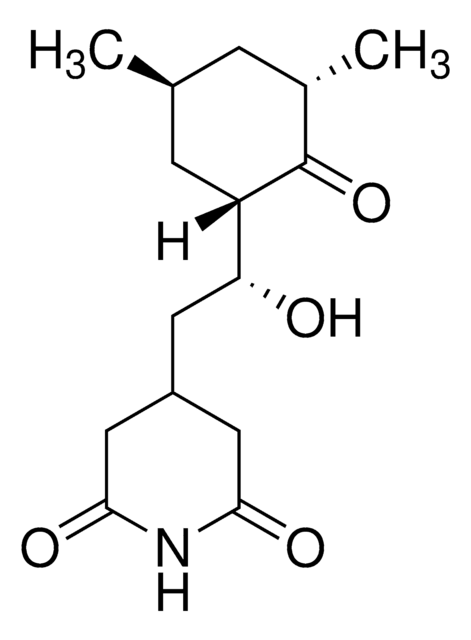
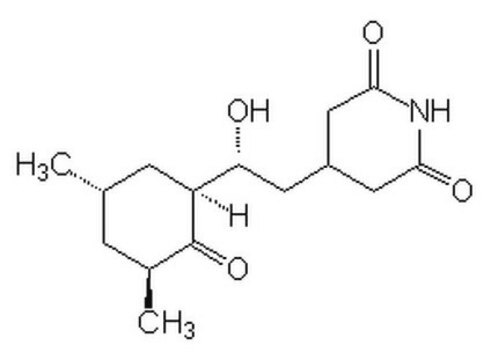
3-[2-(3,5-Dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]glutarimide, Actidione, Naramycin A
About This Item
Recommended Products
biological source
microbial
Quality Level
Assay
≥94% (TLC)
form
powder
storage condition
(Tightly closed. Dry. Keep in a well-ventilated place. Keep locked up or in an area accessible
only to qualified or authorized persons.)
color
white to off-white
solubility
ethanol: soluble, clear to hazy
antibiotic activity spectrum
fungi
yeast
Mode of action
protein synthesis | interferes
storage temp.
2-8°C
SMILES string
[H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC2CC(=O)NC(=O)C2
InChI
1S/C15H23NO4/c1-8-3-9(2)15(20)11(4-8)12(17)5-10-6-13(18)16-14(19)7-10/h8-12,17H,3-7H2,1-2H3,(H,16,18,19)/t8-,9-,11-,12+/m0/s1
InChI key
YPHMISFOHDHNIV-FSZOTQKASA-N
Gene Information
human ... FKBP1A(2280) , PIN1(5300)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
In cell biology and biochemical research, Cycloheximide showcases a dual nature concerning Apoptosis Induction, inducing or inhibiting apoptosis depending on the cell type. Its rapid and reversible effects make it an ideal choice for studying cellular processes and determining protein half-life. Cycloheximide also finds extensive applications in biomedical research, where it inhibits protein synthesis in eukaryotic cells studied in vitro (outside of organisms) and its effects are rapidly reversed by simply removing it. This makes Cycloheximide a go-to choice for exploring cell biology, biomedical and biochemical research, offering precise control and versatility in experiments.
Application
- In yeast strains, cycloheximide has been used as a protein synthesis inhibitor in the cycloheximide chase experiment.
- It has been used to inhibit translation in mammalian cells.
- It has been used to suppress fungal growth.
Biochem/physiol Actions
Activity Spectrum: Active against yeast and fungi like Candida, Aspergillus, Saccharomyces, Penicillium
Features and Benefits
- High-quality antibiotic suitable for multiple research applications
- Commonly used in Cell Biology and Biochemical applications
Other Notes
comparable product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
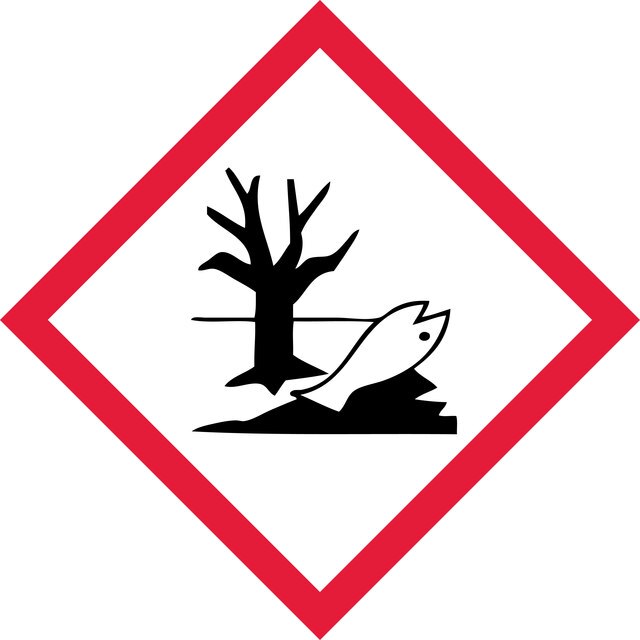
Acute Tox. 2 Oral - Aquatic Chronic 2 - Muta. 2 - Repr. 1B
Storage Class Code
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service