A6680
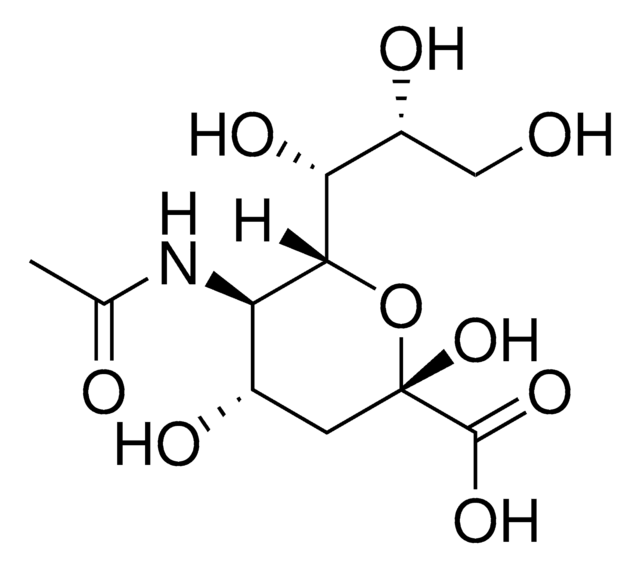
N-Acetylneuraminic Acid Aldolase from microorganisms
lyophilized powder, ≥20 units/mg protein (biuret)
Synonym(s):
N-Acetylneuraminate Pyruvate Lyase, N-Acetylneuraminic Acid Lyase, NANA Aldolase, Sialic Aldolase
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
CAS Number:
Beilstein:
2697172
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥20 units/mg protein (biuret)
mol wt
~98 kDa
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
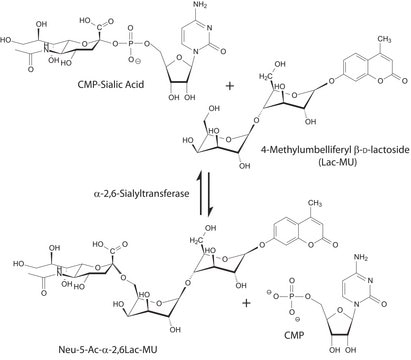
This enzyme is useful for enzymatic determination of N-acetylneuraminic acid and sialic acid when
coupled with the related enzymes in clinical analysis.
For industrial use, this enzyme is useful for enzymatic synthesis of sialic acid.
coupled with the related enzymes in clinical analysis.
For industrial use, this enzyme is useful for enzymatic synthesis of sialic acid.
Used in the Sialic Acid Quantification Kit, SIALIC-Q
Physical properties
Isoelectric point: 4.6 ± 0.1
Michaelis constant: 2.5 x 10‾3M (N-Acetylneuraminic acid)
Structure: 3 subunits (approx. 35,000) per mol of enzyme
Inhibitors: p-Chloromercuribenzoate, sodium dodecyl sulfact, Hg++, Ag+
Optimum pH: 7.5– 8.0
Optimum temp: 70°C
pH Stability: pH 6.0–9.0 (10°C, 25hr)
Thermal stability: Below 65°C (pH 7.5, 30 min)
Michaelis constant: 2.5 x 10‾3M (N-Acetylneuraminic acid)
Structure: 3 subunits (approx. 35,000) per mol of enzyme
Inhibitors: p-Chloromercuribenzoate, sodium dodecyl sulfact, Hg++, Ag+
Optimum pH: 7.5– 8.0
Optimum temp: 70°C
pH Stability: pH 6.0–9.0 (10°C, 25hr)
Thermal stability: Below 65°C (pH 7.5, 30 min)
Unit Definition
One unit will release 1.0 μmole of pyruvate from NANA per min at pH 7.7 at 37 °C.
Physical form
Lyophilized powder containing mannitol and EDTA
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vera Zimmermann et al.
Applied microbiology and biotechnology, 76(3), 597-605 (2007-07-03)
In this work, a model describing the complete enzyme catalysed synthesis of N-acetylneuraminic acid (Neu5Ac) from N-acetyl-D-glucosamine (GlcNAc) is presented. It includes the combined reaction steps of epimerisation from GlcNAc to N-acetyl-D-mannosamine (ManNAc) and the aldol condensation of ManNAc with
Wen-liu Yang et al.
Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences, 39(1), 57-63 (2010-02-23)
To obtain the Escherichia coli strains expressing N-Acetyl-D-neuraminic acid aldolase (Neu5Ac aldolase). The gene (nanA) coding Neu5Ac aldolase was cloned from Escherichia coli C600, and the recombinant plasmid was sequenced and expressed in Escherichia coli. Sequencing data revealed that the
Jozef Nahálka et al.
Journal of biotechnology, 134(1-2), 146-153 (2008-03-04)
The propensity of a recombinant protein produced in bacteria to aggregate has been assumed to be unpredictable, and inclusion bodies have been thought of as wasted cell material. However, a target protein can be purposely driven to inclusion bodies, which
Yanhong Li et al.
Applied microbiology and biotechnology, 79(6), 963-970 (2008-06-04)
Sialic acid aldolases or N-acetylneuraminate lyases (NanAs) catalyze the reversible aldol cleavage of N-acetylneuraminic acid (Neu5Ac) to form pyruvate and N-acetyl-D: -mannosamine (ManNAc). A capillary electrophoresis assay was developed to directly characterize the activities of NanAs in both Neu5Ac cleavage
Hee Gon Jeong et al.
Infection and immunity, 77(8), 3209-3217 (2009-06-03)
N-acetylneuraminic acid (Neu5Ac, sialic acid) could provide a good substrate for enteropathogenic bacteria in the intestine, when the bacteria invade and colonize in human gut. In order to analyze the role of Neu5Ac catabolism in Vibrio vulnificus pathogenesis, a mutant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service