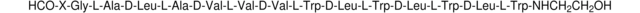A4665
Alamethicin from Trichoderma viride
≥98% (HPLC)
Synonym(s):
Antibiotic U-22324
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
antibiotic activity spectrum
Gram-positive bacteria
Mode of action
cell membrane | interferes
storage temp.
2-8°C
SMILES string
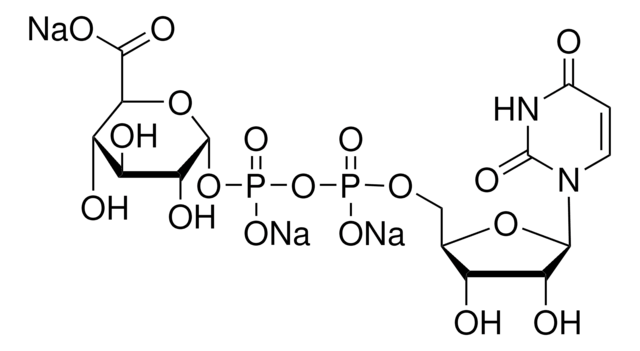
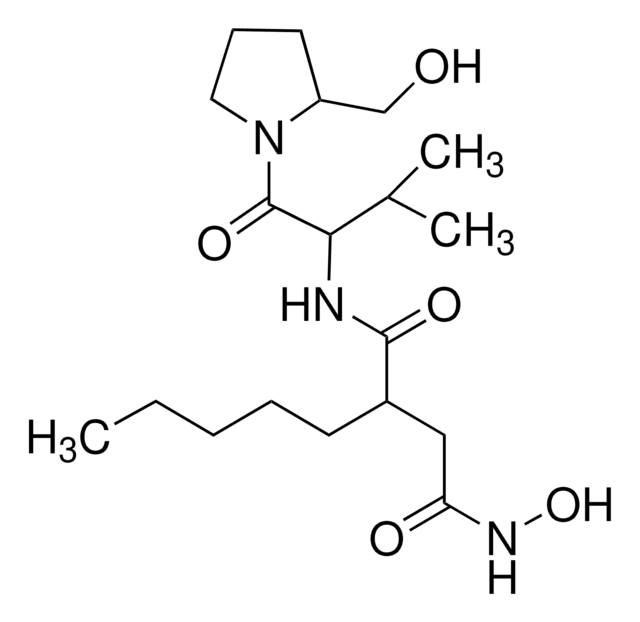
CC(C)C[C@H](NC(=O)CNC(=O)C(C)(C)NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@@H]1CCCN1C(=O)C(C)(C)NC(C)=O)C(C)C)C(=O)NC(C)(C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NC(C)(C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)Cc3ccccc3
InChI
1S/C92H150N22O25/c1-47(2)43-58(72(127)108-92(24,25)84(139)113-41-29-33-59(113)73(128)103-65(48(3)4)75(130)111-90(20,21)82(137)112-89(18,19)80(135)102-56(37-40-64(120)121)70(125)101-55(35-38-61(93)117)69(124)98-54(46-115)44-53-31-27-26-28-32-53)99-63(119)45-95-77(132)85(10,11)110-76(131)66(49(5)6)104-81(136)88(16,17)107-71(126)57(36-39-62(94)118)100-67(122)50(7)96-78(133)86(12,13)106-68(123)51(8)97-79(134)87(14,15)109-74(129)60-34-30-42-114(60)83(138)91(22,23)105-52(9)116/h26-28,31-32,47-51,54-60,65-66,115H,29-30,33-46H2,1-25H3,(H2,93,117)(H2,94,118)(H,95,132)(H,96,133)(H,97,134)(H,98,124)(H,99,119)(H,100,122)(H,101,125)(H,102,135)(H,103,128)(H,104,136)(H,105,116)(H,106,123)(H,107,126)(H,108,127)(H,109,129)(H,110,131)(H,111,130)(H,112,137)(H,120,121)/t50-,51-,54+,55-,56-,57-,58-,59-,60-,65-,66-/m0/s1
InChI key
LGHSQOCGTJHDIL-SLKIUSOBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to examine the effect of glutamate transporters on the Na+/K+-ATPase dependent extracellular K+ transient during neuronal activity
- to investigate the formation of anion-permeable channels in planar lipid bilayers by magainin I obtained from Xenopus skin
- to study the permeability of root apical meristem and epidermis and cellulase induced resistance to Alamethicin in Arabidopsis thaliana
- to study the cytotoxic effects of the phycotoxin okadaic acid and mycotoxins on human intestinal (HT-29) and neuroblastoma (SH-SY5Y) cell lines
- to analyze the structural variations and biological activity of peptaibols obtained from the Longibrachiatum clade belonging to the genus of filamentous fungi Trichoderma
Biochem/physiol Actions
Quality
Sequence
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service