A2169
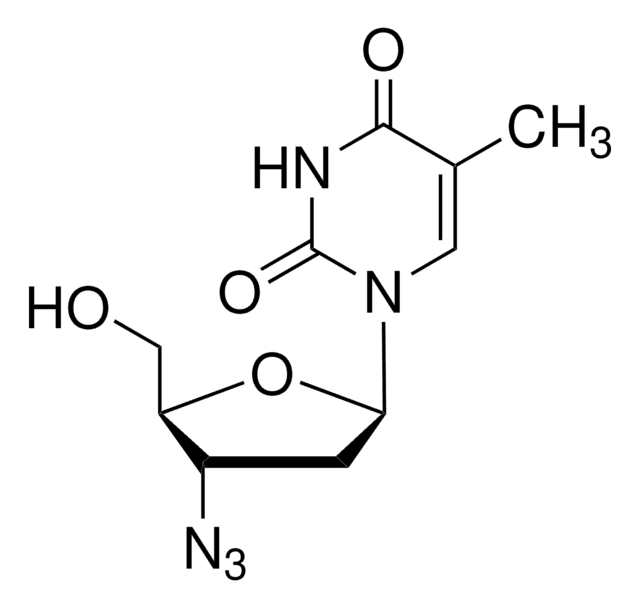
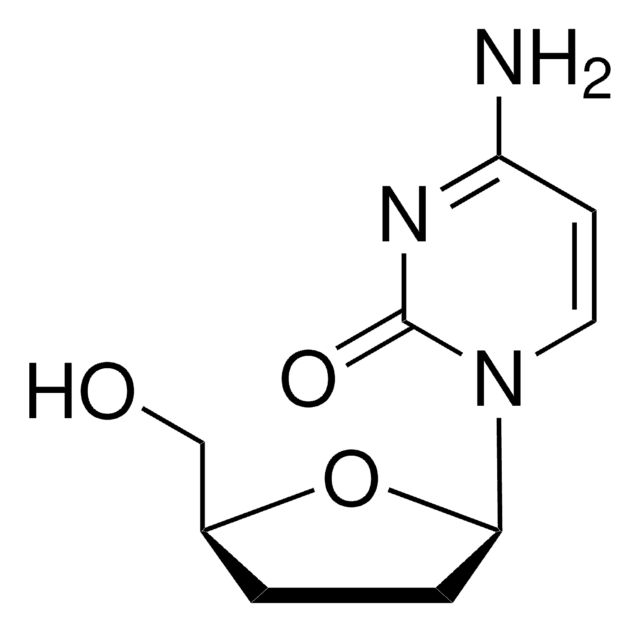
3′-Azido-3′-deoxythymidine
≥98% (HPLC), powder, reverse transcriptase inhibitor
Synonym(s):
AZT, Azidothymidine, ZDV, Zidovudine
About This Item
Recommended Products
product name
3′-Azido-3′-deoxythymidine, ≥98% (HPLC)
Quality Level
Assay
≥98% (HPLC)
form
powder
mp
113-115 °C (lit.)
solubility
H2O: 50 mg/mL
storage temp.
−20°C
SMILES string
CC1=CN([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)C(=O)NC1=O
InChI
1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
InChI key
HBOMLICNUCNMMY-XLPZGREQSA-N
Gene Information
human ... HIVE1(3095)
mouse ... Slc29a1(63959)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Muta. 2
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The CRISPR-Cas9 system is an RNA-guided genome-editing tool that provides researchers a simple, easy, and quick way to modify the genomes of various organisms.
Related Content
We offer agonists, antagonists, modulators and other bioactive small molecules for immune system signaling target identification and validation, as well as a variety of antibiotics, antivirals, and antifungals.
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service