A0810
Endoglycosidase H from Streptomyces plicatus
recombinant, expressed in E. coli, buffered aqueous solution
Synonym(s):
β-N-Acetylglucosaminidase H
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(N-linked)
form
buffered aqueous solution
shipped in
wet ice
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
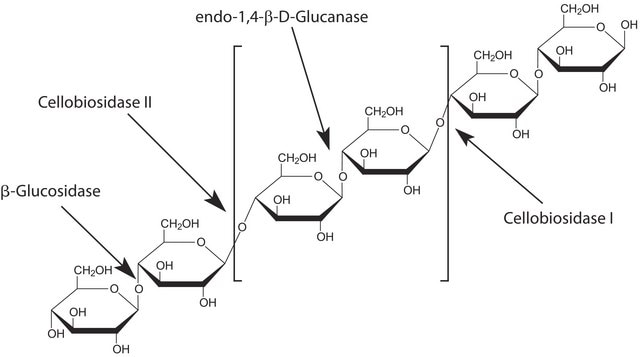
Endoglycosidase H or endo-β-N-acetylglucosaminidase H is an glycohydrolase. It is produced by Streptomyces plicatus and other Streptomyces species.
Biochem/physiol Actions
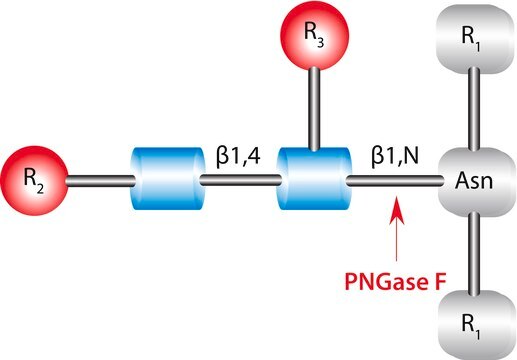
Endoglycosidase H is involved in cleaving the N-linked glycans present between the two N-acetylglucosamine (GlcNAc) residues in the core of the glycan chain in high-mannose sugars.
Unit Definition
One unit will release N-linked oligosaccharides from 1 μmole of denatured ribonuclease B per min at 37 °C at pH 5.5.
Physical form
Solution in 20 mM Tris HCl, pH 7.5, containing 50 mM NaCl, 1 mM EDTA
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
High-Level Expression of Endo-
Freeze H H and Kranz C
Current Protocols in Molecular Biology, 0 17(3) (2010)
Ulla-Maja Bailey et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 923-924, 16-21 (2013-03-05)
Post-translational modification of proteins with glycosylation is of key importance in many biological systems in eukaryotes, influencing fundamental biological processes and regulating protein function. Changes in glycosylation are therefore of interest in understanding these processes and are also useful as
Roger S Zou et al.
Aging, 3(10), 968-984 (2011-10-13)
A distinct conformational transition from the α-helix-rich cellular prion protein (PrPC) into its β-sheet-rich pathological isoform (PrPSc) is the hallmark of prion diseases, a group of fatal transmissible encephalopathies that includes spontaneous and acquired forms. Recently, a PrPSc-like intermediate form
Eric M Rubenstein et al.
The Journal of cell biology, 197(6), 761-773 (2012-06-13)
Little is known about quality control of proteins that aberrantly or persistently engage the endoplasmic reticulum (ER)-localized translocon en route to membrane localization or the secretory pathway. Hrd1 and Doa10, the primary ubiquitin ligases that function in ER-associated degradation (ERAD)
Midori Umekawa et al.
Biochimica et biophysica acta, 1800(11), 1203-1209 (2010-07-22)
An efficient method for synthesizing homogenous glycoproteins is essential for elucidating the structural and functional roles of glycans of glycoproteins. We have focused on the transglycosylation activity of endo-ß-N-acetylglucosaminidase from Mucor hiemalis (Endo-M) as a tool for glycoconjugate syntheses, since
Articles
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service