46051
Esterase from Bacillus stearothermophilus
≥0.2 U/mg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
lyophilized
specific activity
≥0.2 U/mg
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
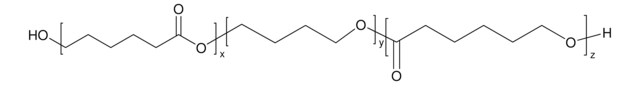
The compound is commonly used for the synthesis of biodiesel and biopolymers, as well as in the production of pharmaceuticals, agrochemicals and flavor compounds.
Biochem/physiol Actions
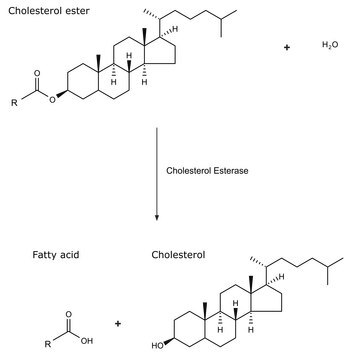
Esterase acts on water-soluble carboxyl esters containing short chain fatty acids .
The esterase catalyzes the transesterification of 1-phenylethanol.
Unit Definition
1 U corresponds to the amount of enzyme which releases 1 μmol 4-nitrophenol per minute at pH 7.0 and 65°C (4-nitrophenyl-n-octanoate as substrate)
Other Notes
Heat stable enzyme
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D.A. Cowan
Enzyme and Microbial Technology, 12, 374-374 (1990)
B Sànchez-Nogué et al.
Environmental science and pollution research international, 20(5), 3480-3488 (2012-12-06)
The common sole, Solea solea (Linneus, 1758), and the Senegalese sole, Solea senegalensis (Kaup, 1858), are two important commercial species that coexist in the NW Mediterranean. In order to assess the species' ability to respond to chemical insults, a comparison
Marie C Fortin et al.
Drug metabolism and disposition: the biological fate of chemicals, 41(2), 326-331 (2012-12-12)
Studies on therapeutic drug disposition in humans have shown significant alterations as the result of pregnancy. However, it is not known whether pesticide metabolic capacity changes throughout pregnancy, which could affect exposure of the developing brain. We sought to determine
XieMei Tang et al.
Critical reviews in eukaryotic gene expression, 22(3), 179-187 (2012-11-13)
Tuberculosis remains one of the most prevalent and deadly infectious diseases, largely due to the emergence of multidrug-resistant and extensive drug-resistant Mycobacterium tuberculosis, especially the coinfection with HIV. Mycobacterium Ag85 complex (Ag85A, B, and C), with a carboxylesterase consensus sequence
Zhe-Yi Hu et al.
Analytical and bioanalytical chemistry, 405(5), 1695-1704 (2012-12-15)
Dabigatran etexilate (DABE) is an oral prodrug that is rapidly converted by esterases to dabigatran (DAB), a direct inhibitor of thrombin. To elucidate the esterase-mediated metabolic pathway of DABE, a high-performance liquid chromatography/mass spectrometry based metabolite identification and semi-quantitative estimation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service