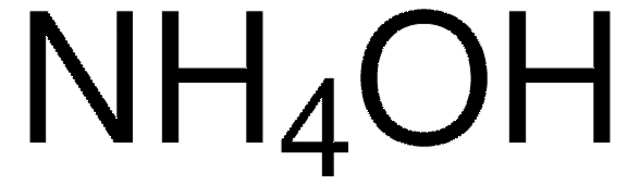338818
Ammonium hydroxide solution
28% NH3 in H2O, ≥99.99% trace metals basis
Synonym(s):
Ammonia aqueous, Ammonia water
About This Item
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
liquid
expl. lim.
27 %
concentration
28% NH3 in H2O
pH
11.7 (20 °C)
density
0.9 g/mL at 25 °C (lit.)
0.9 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[NH4+].[OH-]
InChI
1S/H3N.H2O/h1H3;1H2
InChI key
VHUUQVKOLVNVRT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A medium to prepare magnetite (Fe3O4) nanoparticles via a modified controlled chemical coprecipitation method using divalent or trivalent iron salts as iron precursors.
- A reagent in ammonolysis.
- A solvent to synthesize 1, 4 dihydropyridines via one-pot condensation of an aldehyde and alkyl acetoacetate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Protocols
Separation of Tartrazine; Amaranth; Indigo carmine; New Coccine; Sunset Yellow FCF; Allura Red AC; Fast Green FCF; Erioglaucine disodium salt; Erythrosin B sodium salt; Phloxine B; Rose bengal
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service