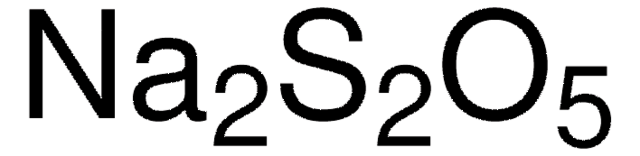All Photos(3)
About This Item
Linear Formula:
KIO3
CAS Number:
Molecular Weight:
214.00
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
grade
reagent grade
Quality Level
Assay
≥98%
form
powder
mp
560 °C (lit.)
density
3.93 g/mL at 25 °C (lit.)
SMILES string
[K+].[O-]I(=O)=O
InChI
1S/HIO3.K/c2-1(3)4;/h(H,2,3,4);/q;+1/p-1
InChI key
JLKDVMWYMMLWTI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
KIO3 can be used as a substitute of KI in radiation protection. A kinetic study of thermal degradation of KIO3 by γ-rays suggests that rate of decomposition increases while activation energy decreases upon irradiation.
Application
Potassium iodate (KIO3) may be used as an oxidizing agent in the estimation of hydrolyzable tannins (gallotannins and ellagitannins) in plant samples. It may also be used in the preparation of polyaniline, poly(o-anisidine), poly[aniline-co-(o-anisidine) and TiO2 nanoparticles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 2
WGK
WGK 1
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mechanistic study of osmium (VIII) promoted oxidation of crotonic acid by aqueous alkaline solution of potassium iodate
Singh B, et al.
Indian Journal of Chemistry, 54, 1387-1393 (2015)
Thermal decomposition kinetics of potassium iodate.
Muraleedharan K.
Journal of Thermal Analysis and Calorimetry, 114(2), 491-496 (2013)
Synthesis and properties of polyaniline, poly (o-anisidine), and poly [aniline-co-(o-anisidine)] using potassium iodate oxidizing agent
Neetika M, et al.
High Performance Polymers (2016)
Yawen Wang et al.
Journal of colloid and interface science, 430, 31-39 (2014-07-07)
In this paper, we report a novel polyol process to synthesize highly water-dispersible anatase titanium dioxide (TiO2) nanoparticles (∼5 nm) by the introduction of inorganic oxidizing agent--KIO3. The obtained TiO2 nanoparticles are well dispersible in water at pH≥5.0 and the
Paul W Hartzfeld et al.
Journal of agricultural and food chemistry, 50(7), 1785-1790 (2002-03-21)
A widely used method for analyzing hydrolyzable tannins afer reaction with KIO(3) has been modified to include a methanolysis step followed by oxidation with KIO(3). In the new method, hydrolyzable tannins (gallotannins and ellagitannins) are reacted at 85 degrees C
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service