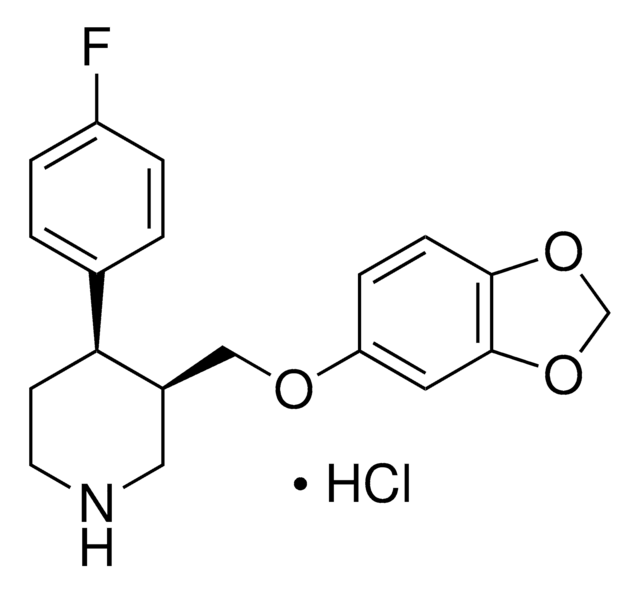
Y0000394
Dipivefrine for system suitability
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
Dipivefrin hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
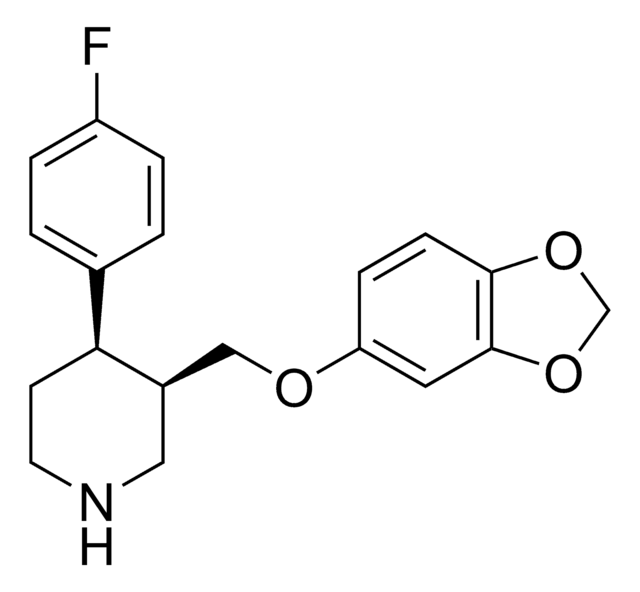
Empirical Formula (Hill Notation):
C19H29NO5 · HCl
CAS Number:
Molecular Weight:
387.90
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
dipivefrine
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C19H29NO5.ClH/c1-18(2,3)16(22)24-14-9-8-12(13(21)11-20-7)10-15(14)25-17(23)19(4,5)6;/h8-10,13,20-21H,11H2,1-7H3;1H
InChI key
VKFAUCPBMAGVRG-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Dipivefrine for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The effect of antiglaucoma drugs on rabbit aqueous humor proteins determined by gel electrophoresis.
M R Reyes et al.
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 14(3), 229-237 (1998-07-22)
Previous studies indicate that there may be differences in the protein composition of the aqueous humor in normal and glaucomatous human eyes. These differences in protein composition and concentration may be due to the topical antiglaucoma medications used to treat
Norio Hori et al.
Clinical autonomic research : official journal of the Clinical Autonomic Research Society, 18(1), 20-27 (2008-02-13)
This study evaluated pupillary postganglionic autonomic dysfunction and its relationship to visual disturbance in idiopathic Parkinson's disease (PD). Pupillary sensitivity was examined in relation to a parasympathomimetic agent [0.05% pilocarpine hydrochloride (PL)] and to a sympathomimetic agent [0.02% dipivefrine hydrochloride
I Widengård et al.
The British journal of ophthalmology, 82(4), 404-406 (1998-06-26)
To investigate the effect on intraocular pressure (IOP) and aqueous flare of topical applications of latanoprost and dipivefrin alone or combined. 22 patients with open angle glaucoma or ocular hypertension were included in a 4 week open label study. Median
F Neu
Bulletin de la Societe belge d'ophtalmologie, (304)(304), 71-76 (2007-08-28)
Cystoid macular edema is a known side effect of different systemic and local medications. Nicotinic acid used as a hypolipemiant agent can cause cystoid macular edema. Local adrenergic antiglaucomatous drugs as well as prostaglandin analogs can induce cystoid macular edema
Hojung Choi et al.
Journal of veterinary science, 12(1), 99-101 (2011-03-04)
Color Doppler imaging (CDI) was carried out to evaluate the effects of anti-glaucoma drugs on ophthalmic circulation using CDI-derived resistive index (RI) values. CDI was performed on nine Beagle dogs, and RI values were calculated for the medial long posterior
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service