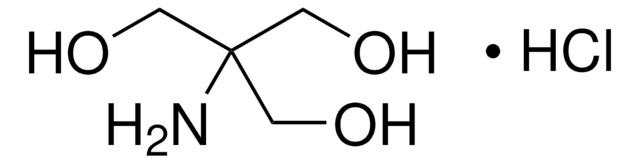
T15760
Trizma® hydrochloride
reagent grade, ≥99%, crystalline
Synonym(s):
TRIS HCl, TRIS hydrochloride, Tris(hydroxymethyl)aminomethane hydrochloride, Tromethane hydrochloride
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
reagent grade
Assay
≥99%
form
crystalline
storage condition
(Tightly closed. Dry)
impurities
<0.5% water
color
white
useful pH range
7.0-9.0
pKa (25 °C)
8.1
mp
~150.7 °C
solubility
water: 1.5 g/mL, clear, colorless
cation traces
Fe: ≤5 ppm
Pb: ≤5 ppm
storage temp.
room temp
SMILES string
Cl.NC(CO)(CO)CO
InChI
1S/C4H11NO3.ClH/c5-4(1-6,2-7)3-8;/h6-8H,1-3,5H2;1H
InChI key
QKNYBSVHEMOAJP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Essential in protein electrophoresis and western blotting techniques, Tris buffers, particularly low ionic strength versions, are employed for protein transfer. They are also commonly used in SDS-PAGE gels, running buffers, and blotting buffers. Additionally, Tris salts, integral to DNA agarose electrophoresis, contribute to buffer preparations like TAE (Tris acetate/EDTA) and TBE (Tris borate/EDTA), playing pivotal roles in biochemistry, protein purification, and electrophoresis applications. Overall, Tris HCl proves to be a versatile and indispensable component, ensuring optimal pH in biological systems for effective biochemical and cell biology research.
Features and Benefits
- Ideal as a Biological buffer for Cell Biology and Biochemical research
- Effective Buffering from pH 7-9 (25 °C) with a pKa of 8.1 (25 °C)
- Tested to confirm low levels of heavy metal contamination, ensuring suitability for various applications
Other Notes
For precise applications, use a carefully calibrated pH meter with a glass/calomel combination electrode.
Legal Information
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service